Corrosion Behavior of Alumina-forming Austenitic Heat Resistant Steel in Supercritical Carbon Dioxide
-
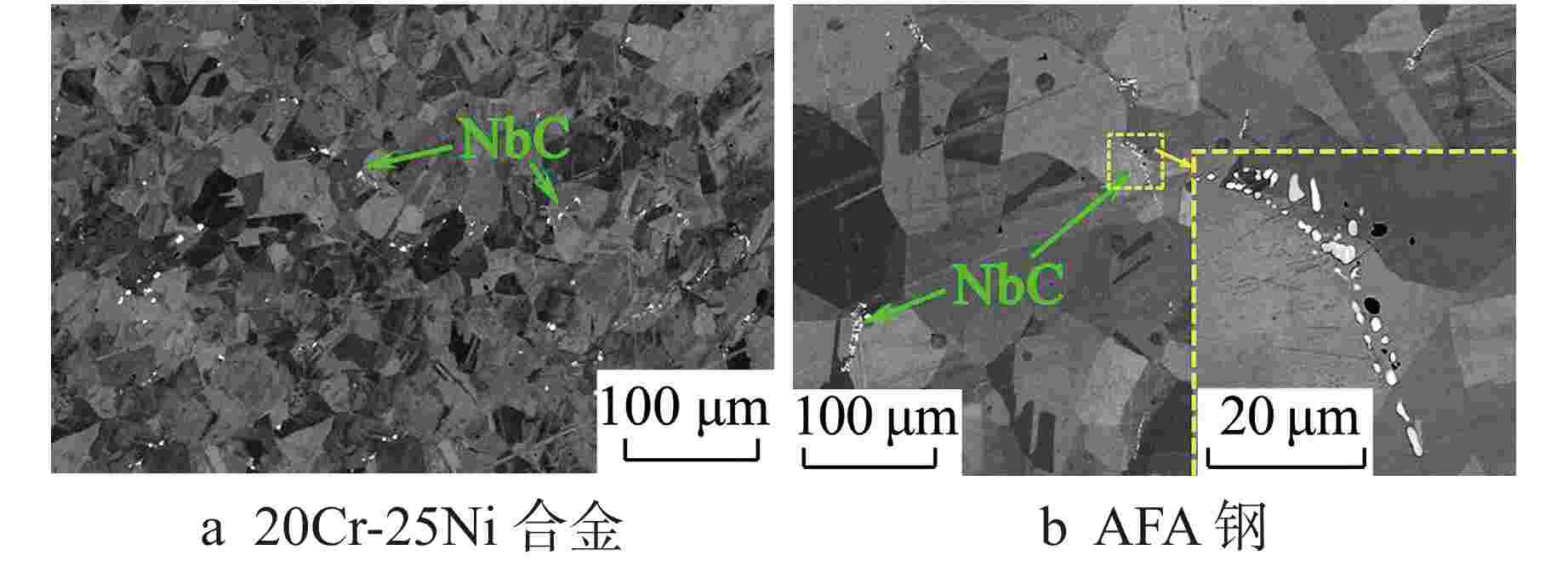
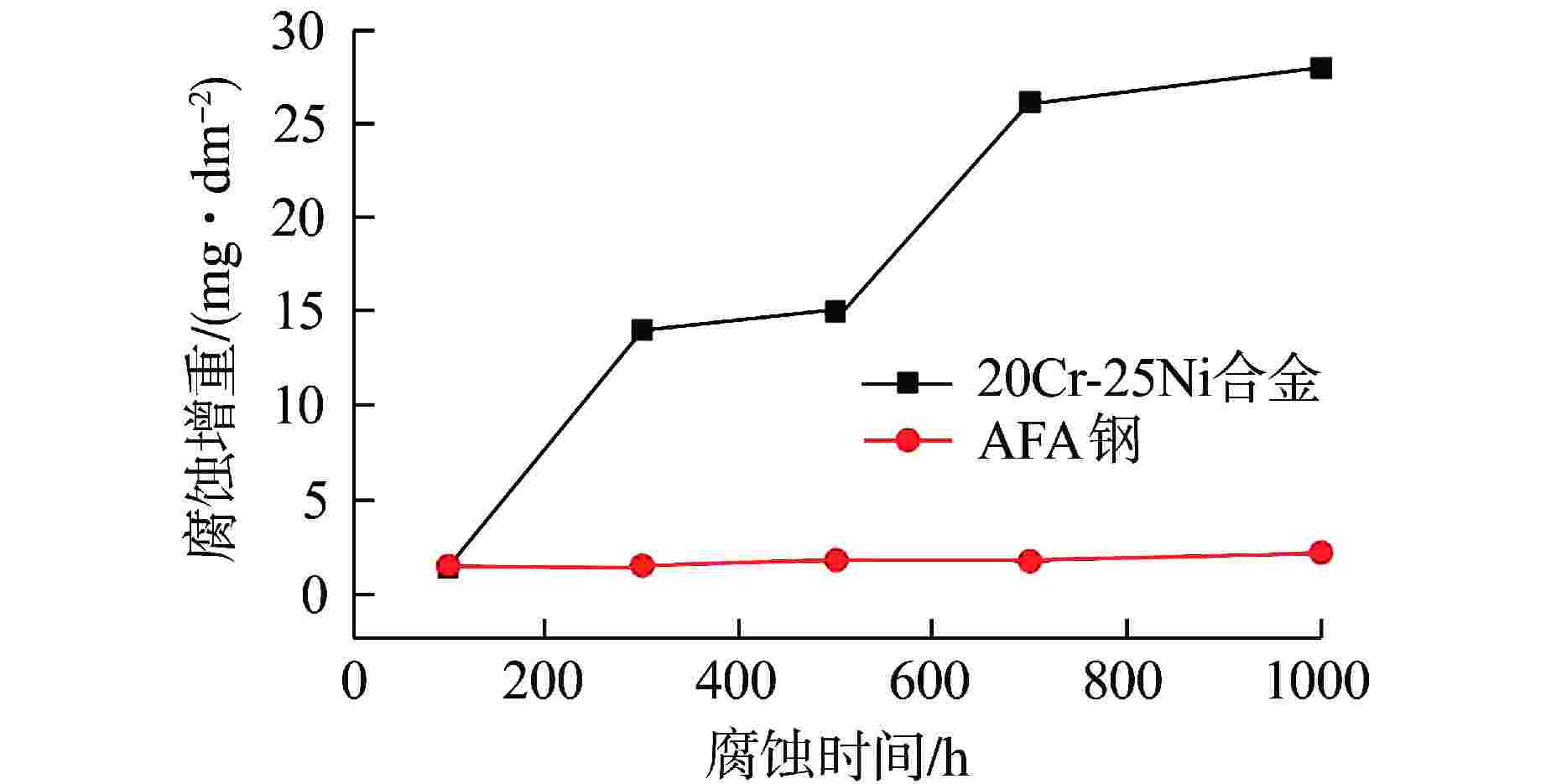
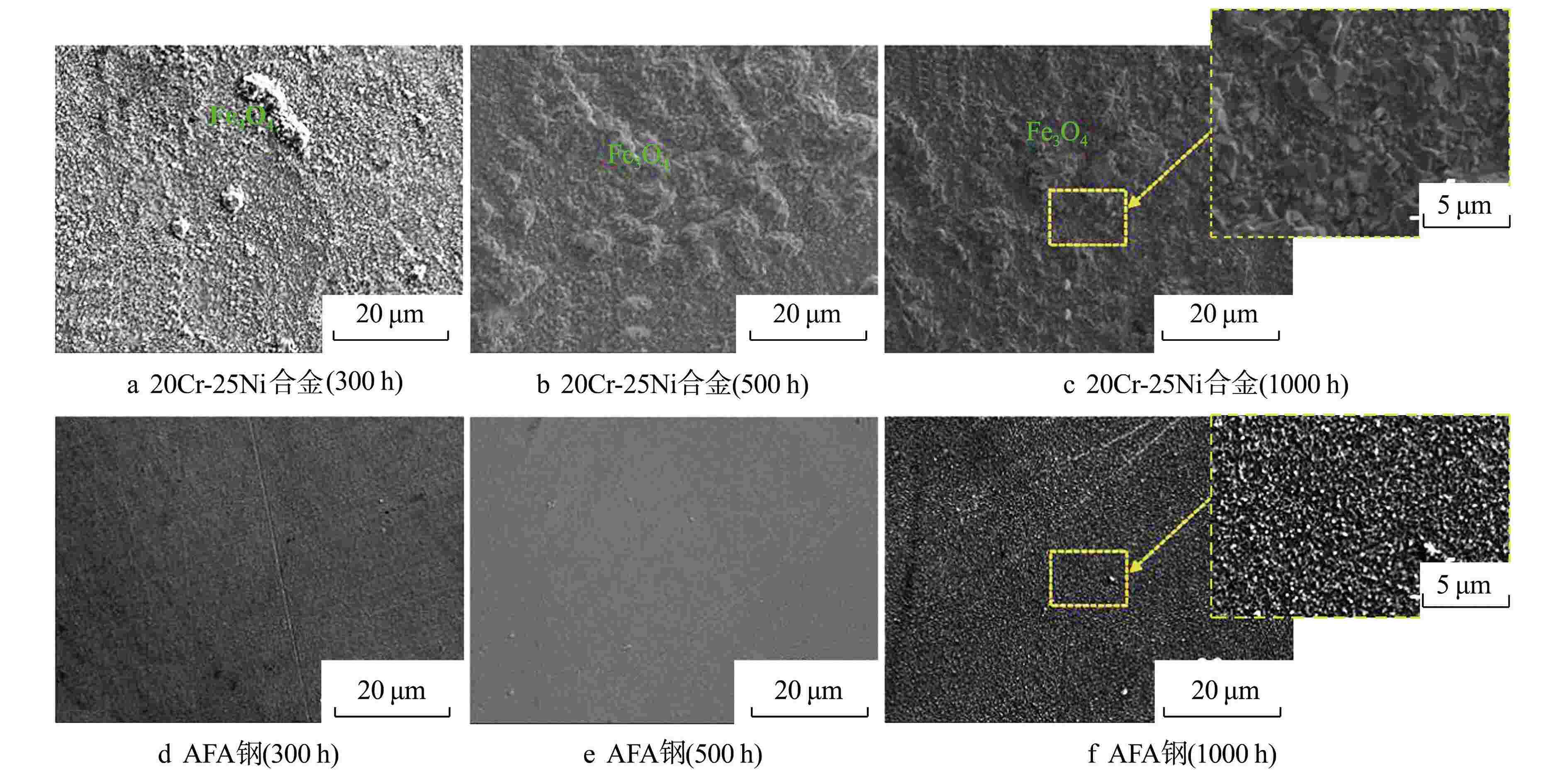
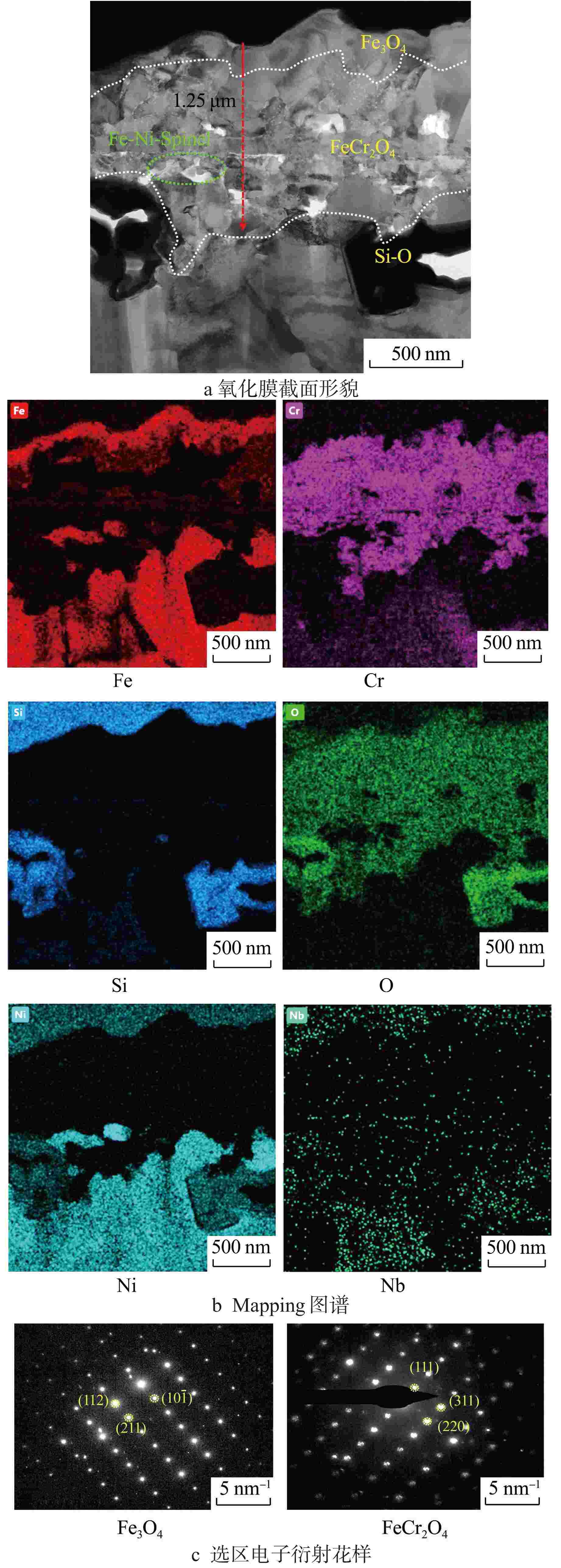
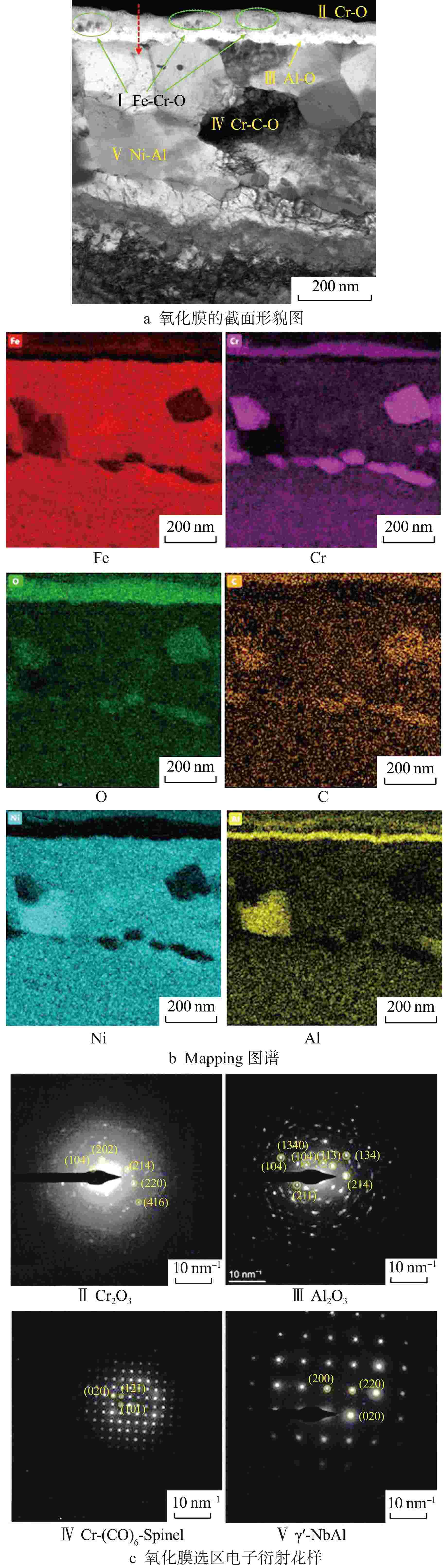
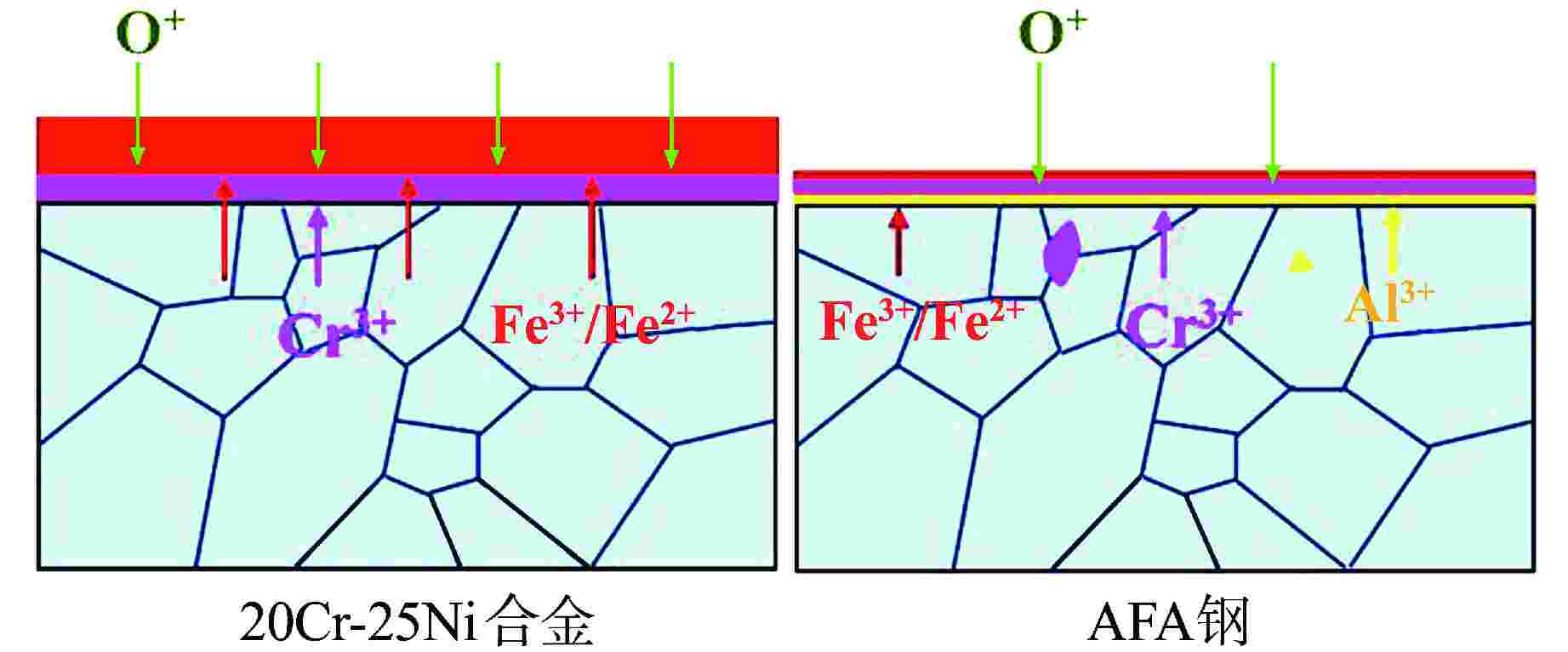
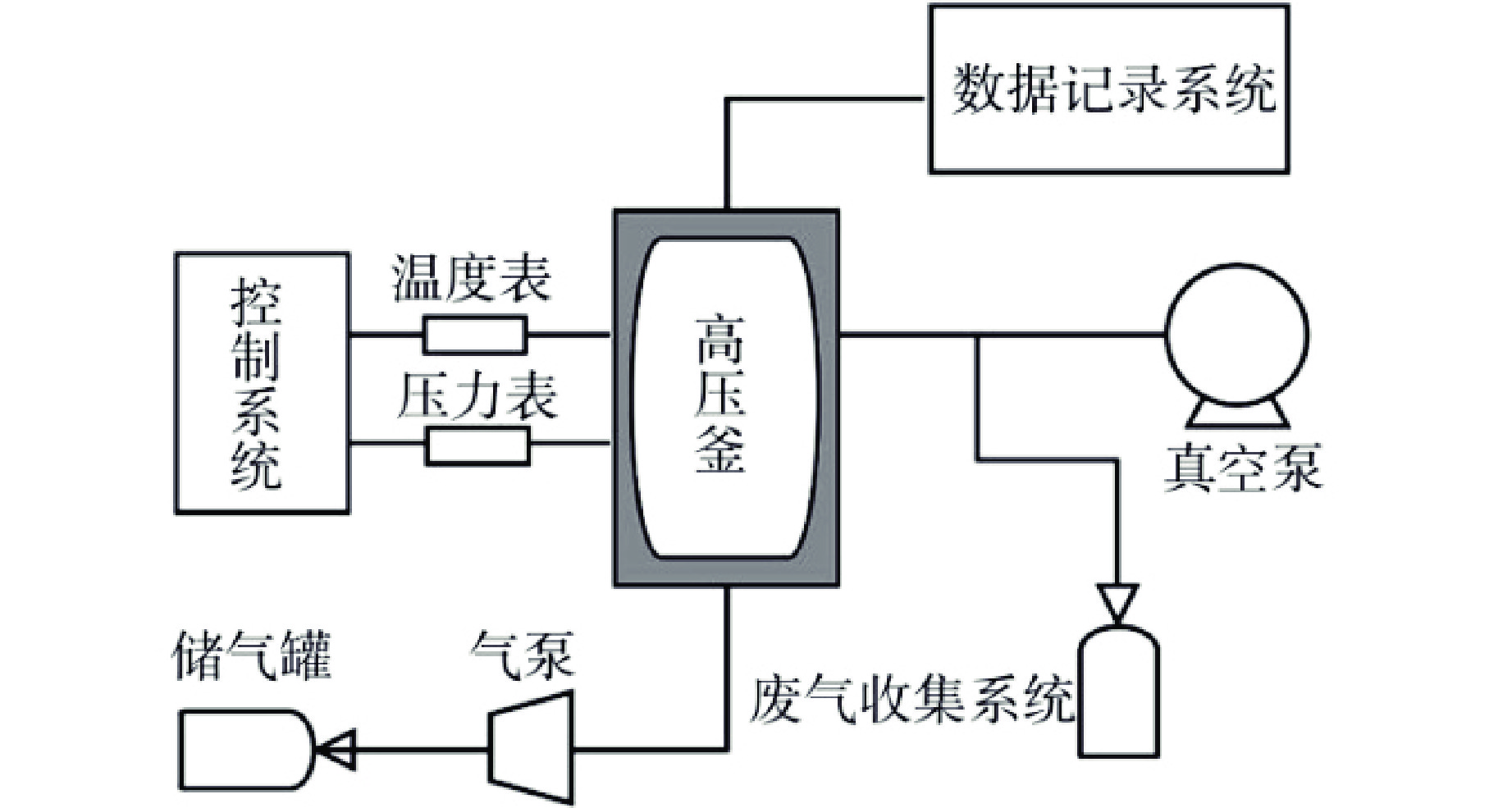
摘要: 研究了20Cr-25Ni合金和一种新型结构材料含铝的奥氏体耐热钢(AFA钢)在600℃/20 MPa的超临界二氧化碳(S-CO2)环境中的腐蚀行为,并对2种合金的氧化膜形貌、成分和结构进行分析。研究发现,20Cr-25Ni合金出现明显的腐蚀增重增长趋势,表现出“抛物线”上升规律;AFA钢腐蚀增重趋势缓慢,腐蚀1000 h后仅为2.11 mg/dm2。20Cr-25Ni合金表面出现粗大的氧化产物,随腐蚀时间延长,AFA钢的氧化膜始终保持致密、连续。通过氧化膜的截面形貌分析发现,20Cr-25Ni合金腐蚀后具有两层氧化膜结构,主要由Fe3O4和FeCr2O4氧化层以及少量尖晶石组成。而AFA钢中出现了3层氧化膜结构,中间和最内层分别为Cr2O3和Al2O3氧化膜,最外层分布了一层不连续的FeCr2O4尖晶石氧化物。由于形成了致密的Al2O3氧化膜,AFA钢的抗腐蚀性能大幅提升。
-
关键词:
- 含铝奥氏体耐热钢(AFA钢) /
- 超临界二氧化碳(S-CO2) /
- 均匀腐蚀 /
- 铝氧化膜
Abstract: The corrosion behavior of Alloy 20Cr-25Ni and a new structural material alumina-forming austenitic heat resistant steel (AFA steel) in supercritical carbon dioxide (S-CO2) environment at 600℃/20 MPa was studied. The morphology, composition, and structure of oxide films of the two alloys were analyzed. It is found that the weight gain curves of the two alloys were in accord with the “parabola” law. The weight of corrosion products increased slowly; it was only 2.11 mg/dm2 after 1000 h. By comparison, the coarse oxide products appeared on the surface of Alloy 20Cr-25Ni and increased with the extension of corrosion time, while oxide film of AFA steel remained dense and continuous. Through the analysis of the cross-sectional morphology of the oxide film, it is found that the Alloy 20Cr-25Ni has a two-layer oxide film structure after corrosion, which is mainly composed of Fe3O4 and FeCr2O4 oxide layer and a small amount of spinel. However, there are three layers of oxide film structure in AFA steel, the middle and inner layers are Cr2O3 and Al2O3 oxide films respectively, and the outer layer is distributed with a discontinuous FeCr2O4 spinel oxide. Due to the formation of dense Al2O3 oxide film, the corrosion resistance of AFA steel is greatly improved. -
表 1 2种合金材料的化学成分
Table 1. Chemical Composition of Two Alloy Materials
材料 元素质量分数/% Ni Cr Al Nb Mo Si C Fe 20Cr-25Ni合金 25 20 0 0.7 2 0.3 0.02 Bal. AFA钢 25 18 3.5 0.6 2 0.3 0.02 Bal. Bal.—其余成分 -
[1] KATO Y, NITAWAKI T, MUTO Y. Medium temperature carbon dioxide gas turbine reactor[J]. Nuclear Engineering and Design, 2004, 230(1-3): 195-207. doi: 10.1016/j.nucengdes.2003.12.002 [2] 黄彦平,王俊峰. 超临界二氧化碳在核反应堆系统中的应用[J]. 核动力工程,2012, 33(3): 21-27. doi: 10.3969/j.issn.0258-0926.2012.03.005 [3] AHN Y, BAE S J, KIM M, et al. Review of supercritical CO2 power cycle technology and current status of research and development[J]. Nuclear Engineering and Technology, 2015, 47(6): 647-661. doi: 10.1016/j.net.2015.06.009 [4] EOH J H, NO H C, YOO Y H, et al. Sodium-CO2 interaction in a supercritical CO2 power conversion system coupled with a sodium fast reactor[J]. Nuclear Technology, 2011, 173(2): 99-114. doi: 10.13182/NT11-A11541 [5] YAMAMOTO Y, BRADY M P, LU Z P, et al. Creep-resistant, Al2O3-forming austenitic stainless steels[J]. Science, 2007, 316(5823): 433-436. doi: 10.1126/science.1137711 [6] YAMAMOTO Y, BRADY M P, LU Z P, et al. Alumina-forming austenitic stainless steels strengthened by laves phase and MC carbide precipitates[J]. Metallurgical and Materials Transactions A, 2007, 38(11): 2737-2746. doi: 10.1007/s11661-007-9319-y [7] 刘珠,郭相龙,王鹏,等. 310S不锈钢在超临界二氧化碳中的腐蚀行为研究[J]. 核动力工程,2020, 41(S1): 183-187. doi: 10.13832/j.jnpe.2020.S1.0183 [8] FURUKAWA T, INAGAKI Y, ARITOMI M. Compatibility of FBR structural materials with supercritical carbon dioxide[J]. Progress in Nuclear Energy, 2011, 53(7): 1050-1055. doi: 10.1016/j.pnucene.2011.04.030 [9] HE L F, ROMAN P, LENG B, et al. Corrosion behavior of an alumina forming austenitic steel exposed to supercritical carbon dioxide[J]. Corrosion Science, 2014, 82: 67-76. doi: 10.1016/j.corsci.2013.12.023 [10] SHI H, JIANU A, WEISENBURGER A, et al. Corrosion resistance and microstructural stability of austenitic Fe-Cr-Al-Ni model alloys exposed to oxygen-containing molten lead[J]. Journal of Nuclear Materials, 2019, 524: 177-190. doi: 10.1016/j.jnucmat.2019.06.043 [11] GIGGINS C S, PETTI F S. Oxidation of Ni-Cr-Al alloys between 1000℃ and 1200℃[J]. Journal of the Electrochemical Society, 1971, 118(11): 1782-1790. doi: 10.1149/1.2407837 [12] CHEN H S, KIM S H, KIM C, et al. Corrosion behaviors of four stainless steels with similar chromium content in supercritical carbon dioxide environment at 650℃[J]. Corrosion Science, 2019, 156: 16-31. doi: 10.1016/j.corsci.2019.04.043 [13] CAO G, FIROUZDOR V, SRIDHARAN K, et al. Corrosion of austenitic alloys in high temperature supercritical carbon dioxide[J]. Corrosion Science, 2012, 60: 246-255. doi: 10.1016/j.corsci.2012.03.029 [14] BRADY M P, KEISER J R, MORE K L, et al. Comparison of short-term oxidation behavior of model and commercial chromia-forming ferritic stainless steels in dry and wet air[J]. Oxidation of Metals, 2012, 78(1-2): 1-16. doi: 10.1007/s11085-012-9289-3 -





 下载:
下载: