Treatment of Simulated Radioactive Cobalt Containing Wastewater by Ultra-low Pressure Reverse Osmosis Process
-
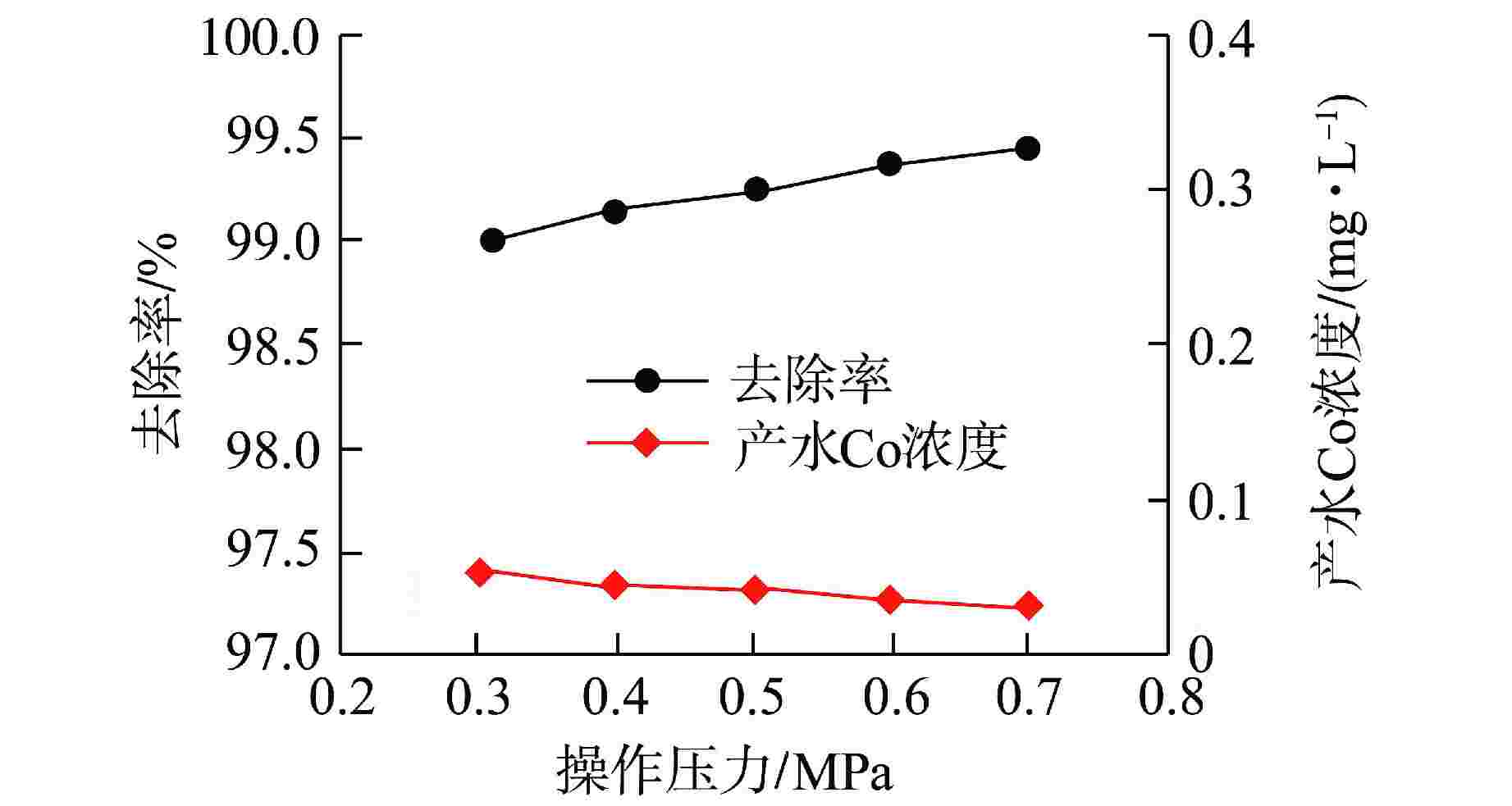
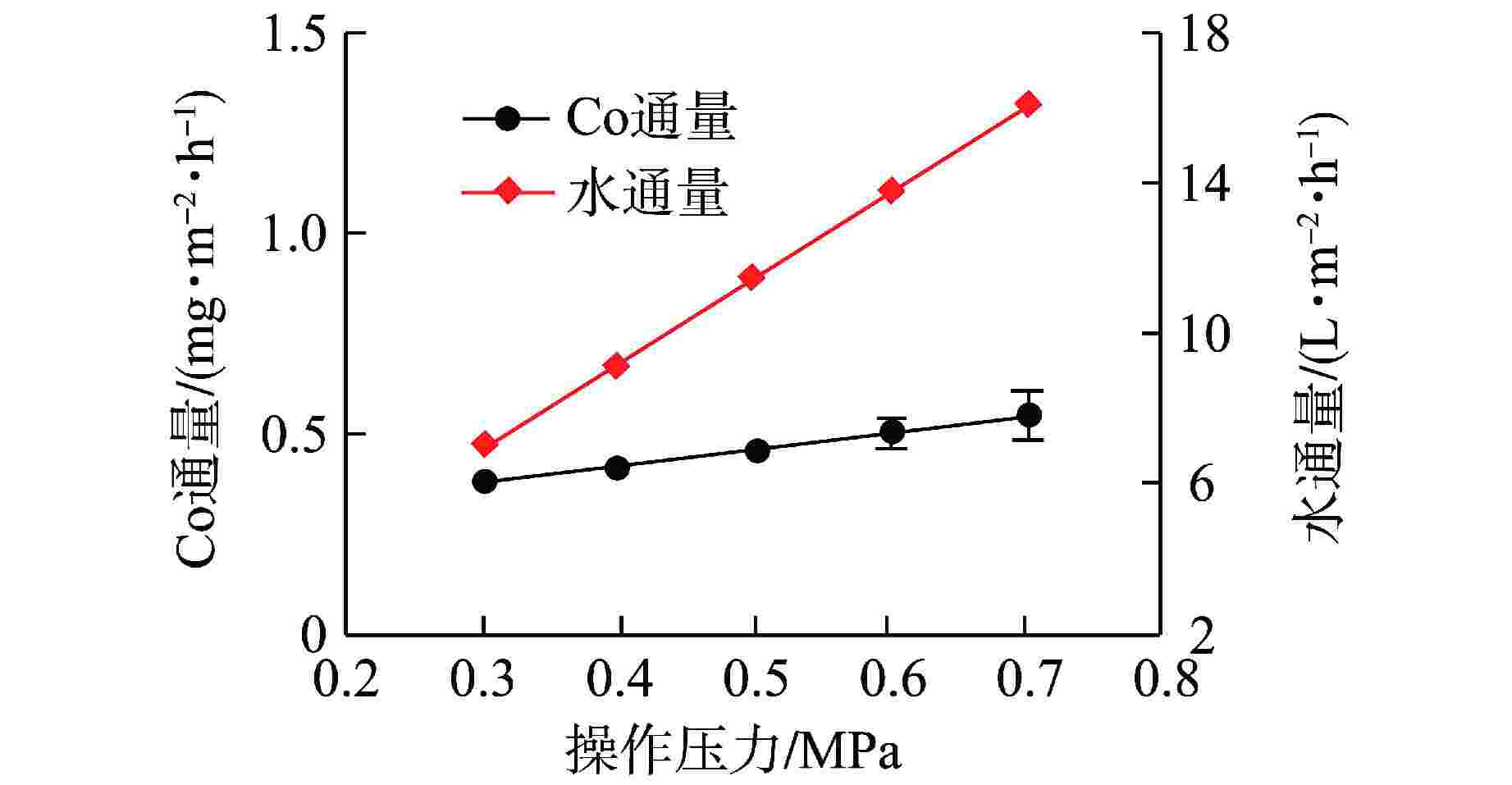
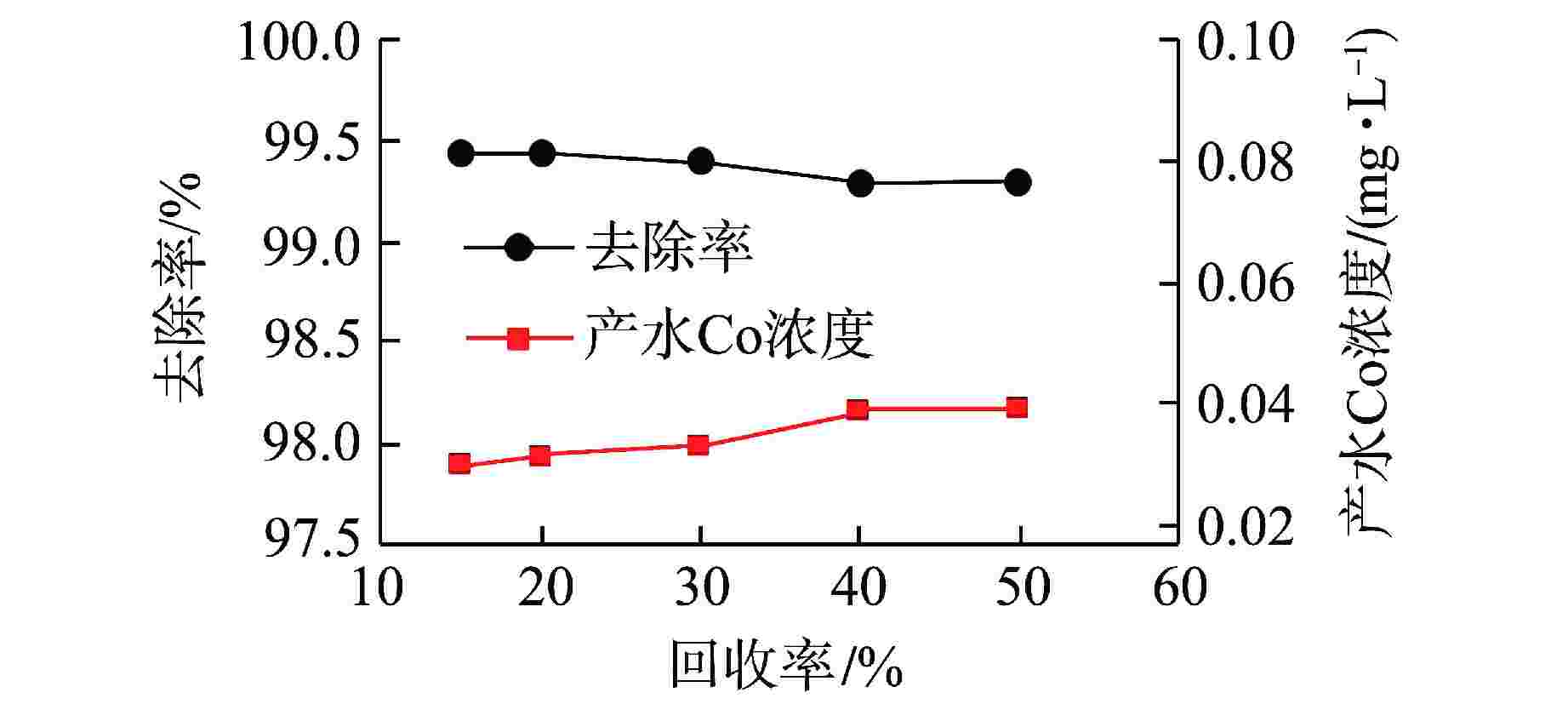
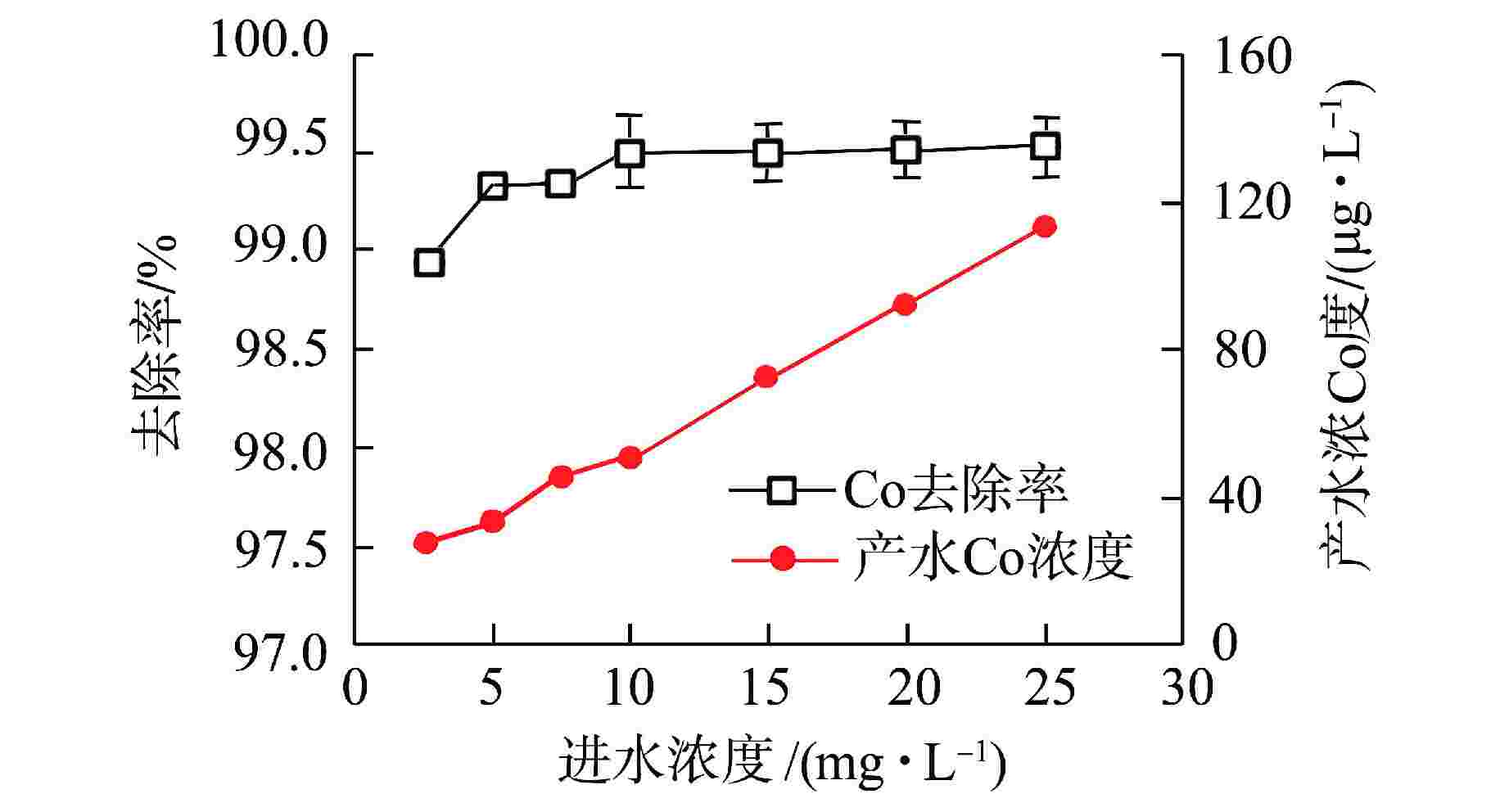
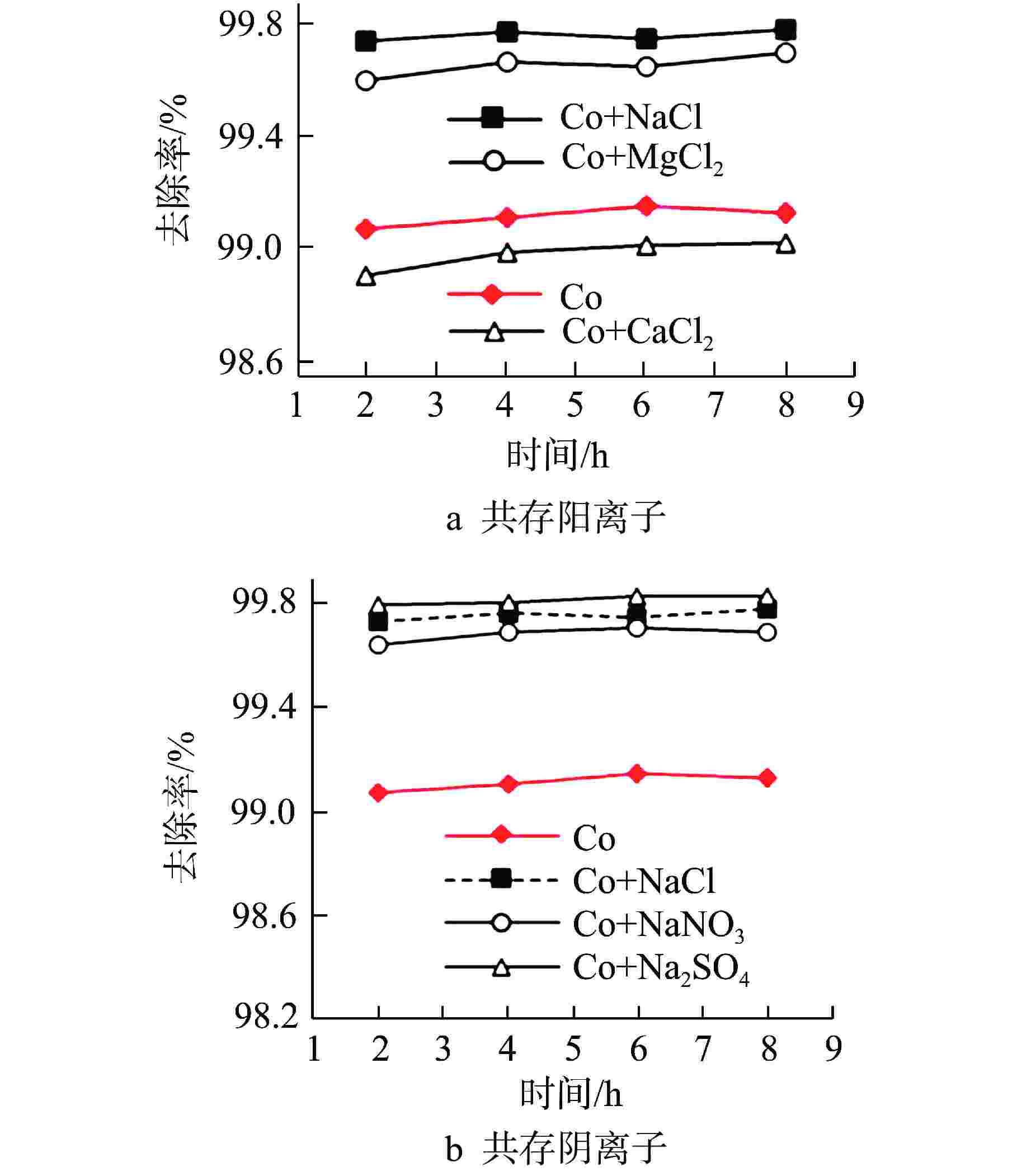
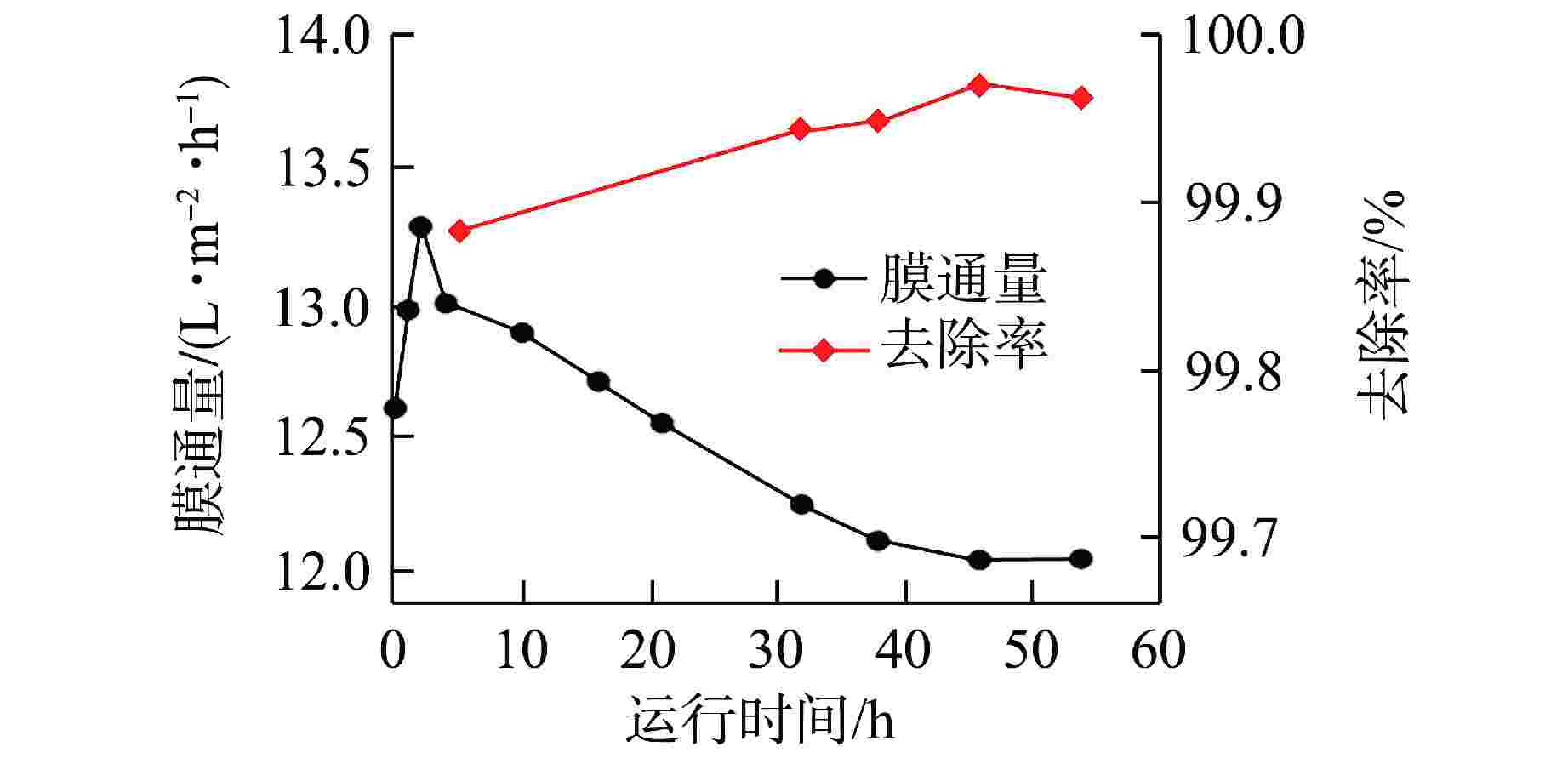
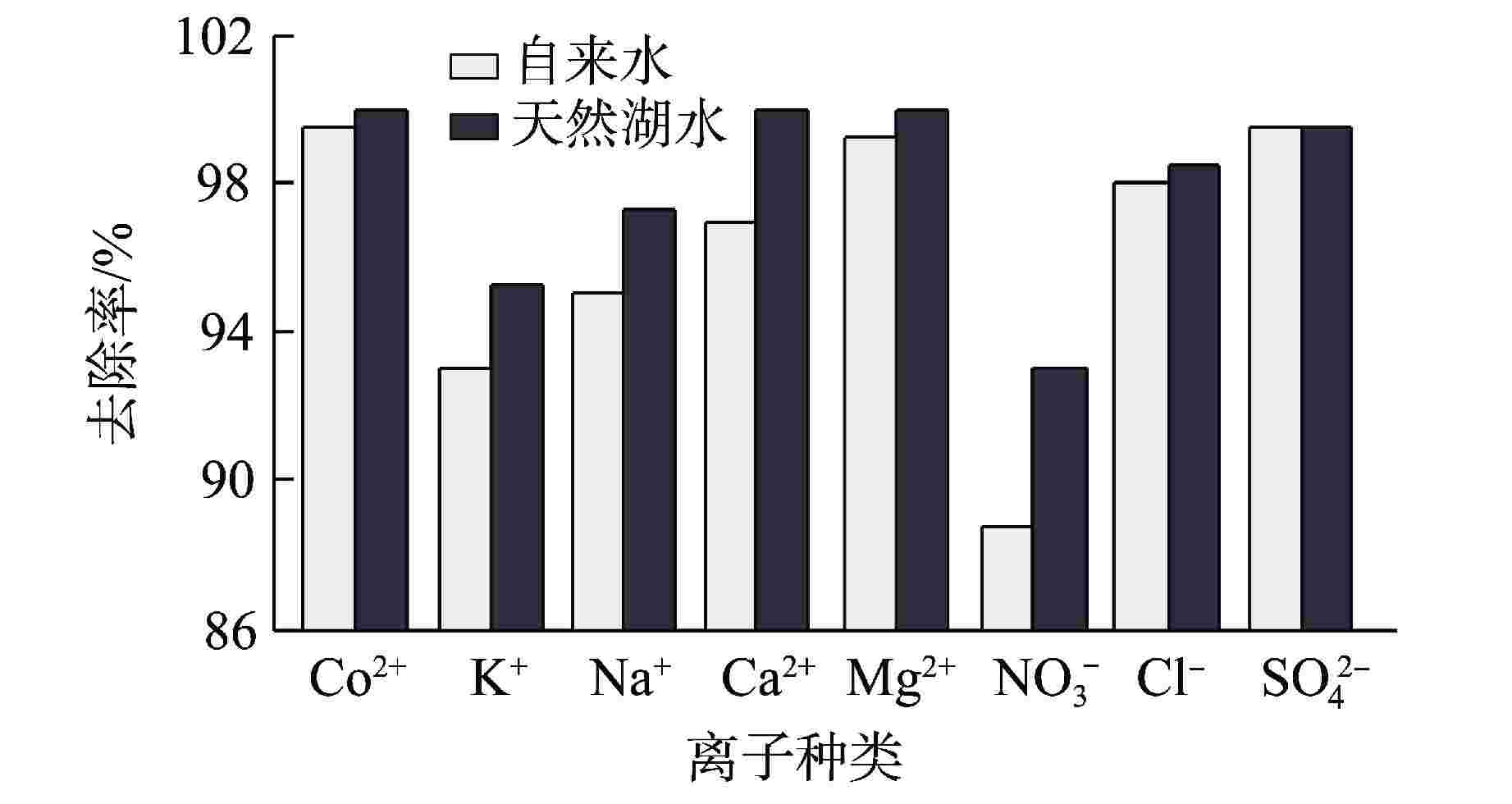
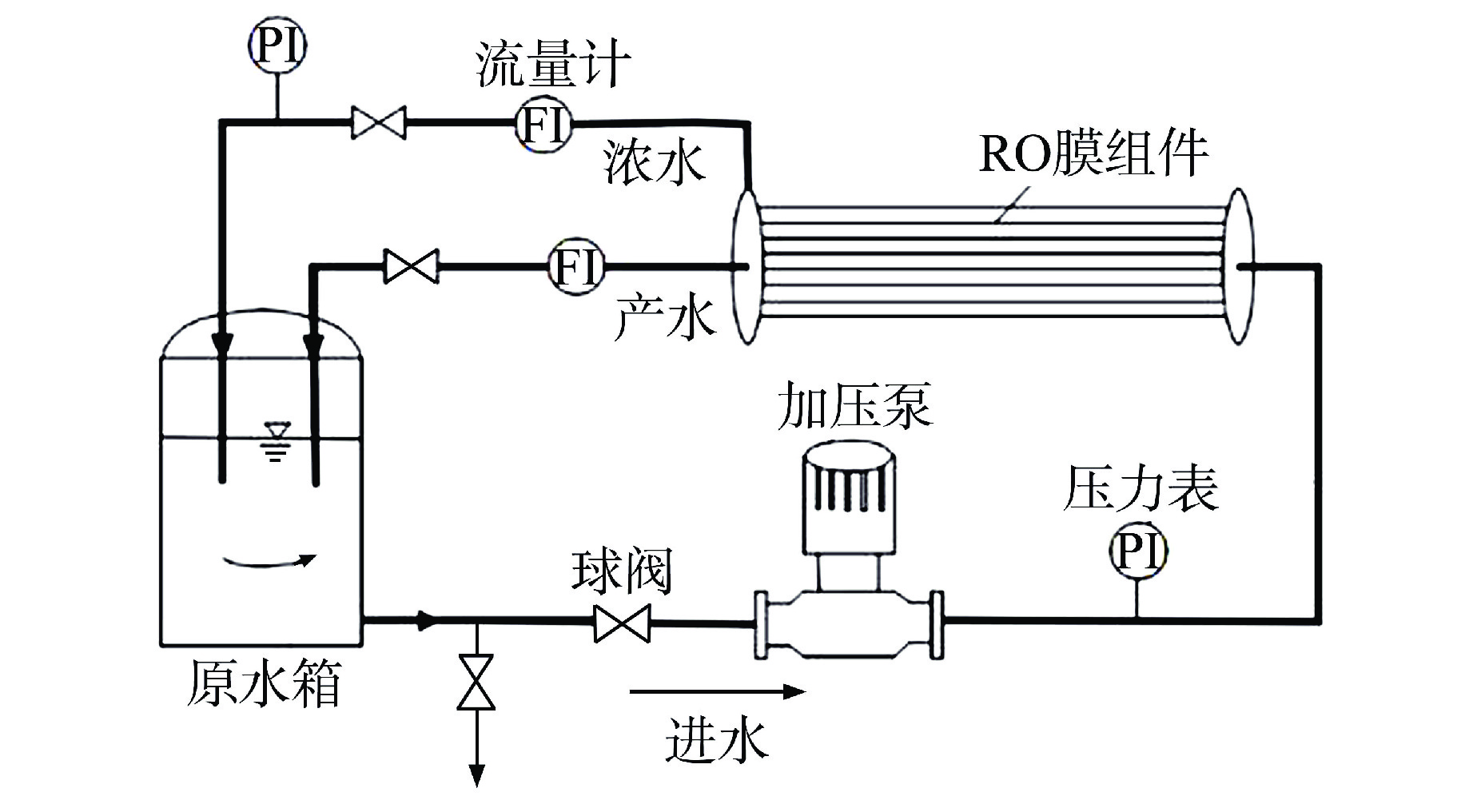
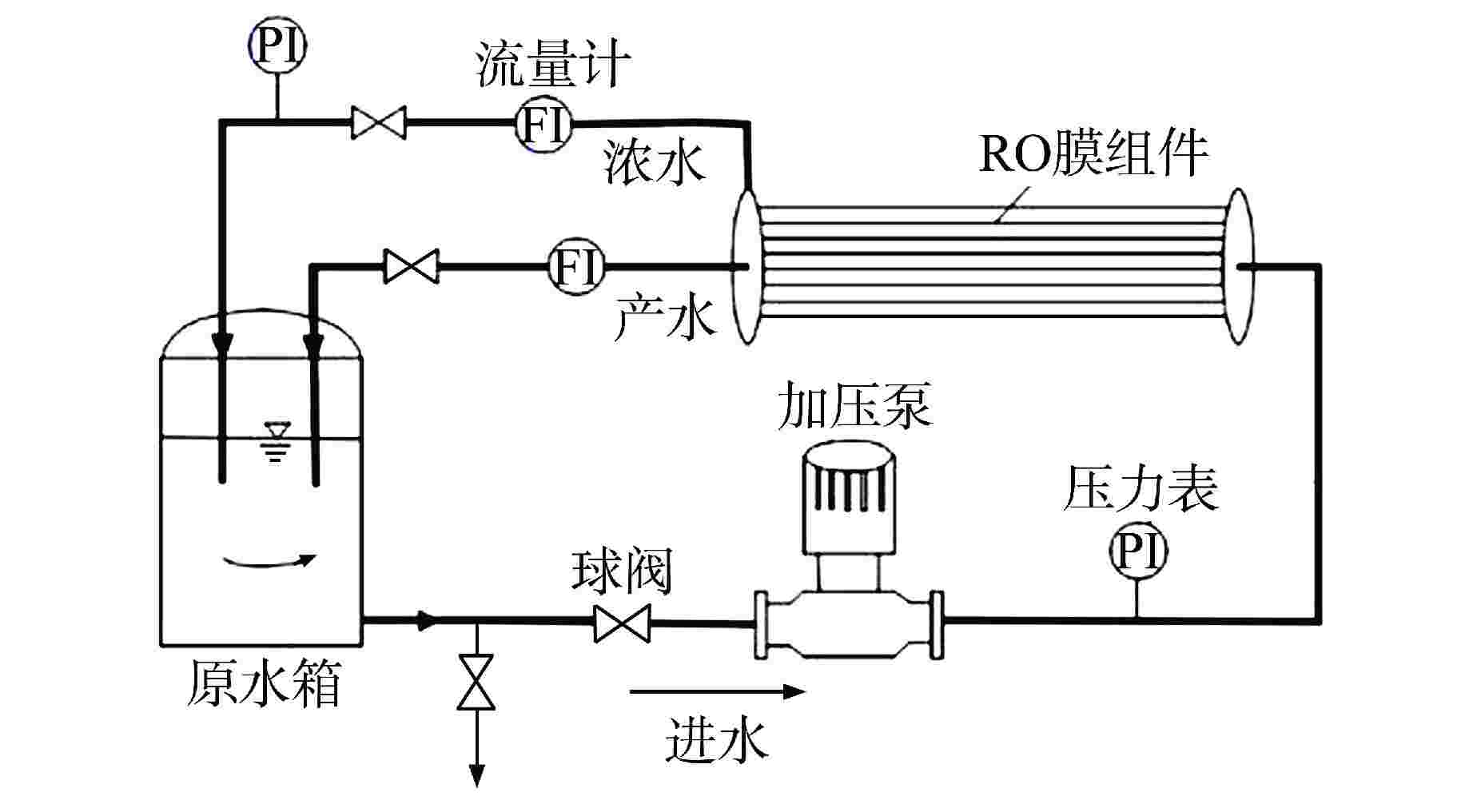
摘要: 核电厂运行、维护和退役产生的放射性废水对社会环境和生命健康安全有严重危害。采用中试装置(144 L/h)研究了超低压反渗透(ULPRO)工艺对模拟放射性废水中目标核素钴(Co)的去除效果,确定了操作压力、回收率、进水浓度、共存离子及天然有机污染物对其影响规律。结果表明,Co去除率与操作压力正相关、与回收率负相关,随进水浓度增大而后恒定在99.5%,10 mg/L为其变化的临界浓度;共存离子仅Ca2+会抑制Co的去除,其他离子的促进作用强弱排序为Na+>Mg2+,SO42−>Cl−>NO3−;有机物污染造成膜通量下降9.4%,但Co去除率增大至99.97%。实验验证了ULPRO工艺处理模拟放射性含Co废水运行效果稳定、去除率高,可为工业应用提供指导。
-
关键词:
- 低水平放射性废水 /
- 超低压反渗透(ULPRO) /
- 膜分离 /
- 钴(Co)
Abstract: The radioactive wastewater produced by the operation, maintenance and decommissioning of nuclear power plants is seriously harmful to the social environment and life and health safety. A pilot plant (144 L/h) is used to study the removal effect of cobalt (Co) from simulated radioactive wastewater by ultra-low pressure reverse osmosis (ULPRO) process. The effects of operating pressure, recovery rate, influent concentration, coexisting ions and natural organic pollutants on it are determined. The results show that the removal rate of Co is positively correlated with the operating pressure and negatively correlated with the recovery rate, the removal rate of Co is constant at 99.5% with the increase of influent concentration, and 10 mg/L is the critical concentration for its change; As for coexisting ions, only Ca2+will inhibit the removal of Co, and the promotion effect of other ions is in the order of Na+>Mg2+, SO42−>Cl−>NO3−; Organic pollution reduces the membrane flux by 9.4%, but the removal rate of Co increases to 99.97%. The experimental results show that the ULPRO process has stable operation effect and high removal rate for the treatment of simulated radioactive wastewater containing Co, which can provide guidance for industrial application. -
表 1 模拟放射性废水水质参数
Table 1. Water Quality Parameters of Simulated Radioactive Wastewater
参数 自来水 天然湖水 pH 8.05 8.85 电导率/(μS·cm−1) 276 1198 浊度/NTU 0.11 0.30① Na+浓度/(mg·L−1) 21.45 162.10 K+浓度/(mg·L−1) 2.12 12.51 Ca2+浓度/(mg·L−1) 20.48 108.35 Mg2+浓度/(mg·L−1) 11.04 47.14 Cl−浓度/(mg·L−1) 12.47 141.80 NO3−浓度/(mg·L−1) 4.23 13.78 SO42−浓度/(mg·L−1) 24.78 178.12 NPOC②/(mg·L−1) 1.40 16.6 注:①使用0.45 μm微滤膜过滤之后的湖水浊度;②NPOC—有机物浓度。 表 2 阳离子水合半径
Table 2. Cation Hydration Radius
离子种类 Co2+ Na+ Mg2+ Ca2+ 水合离子半径/nm 0.423 0.365 0.429 0.412 -
[1] International Atomic Energy Agency. Operating experience with nuclear power stations in member states[Z]. Vienna: IAEA, 2020. [2] International Atomic Energy Agency. Nuclear power reactors in the world[Z]. Vienna: IAEA, 2020. [3] CHOO K H, KWON D J, LEE K W, et al. Selective removal of cobalt species using nanofiltration membranes[J]. Environmental Science & Technology, 2002, 36(6): 1330-1336. [4] STRATHMANN H. Membrane separation processes: current relevance and future opportunities[J]. AIChE Journal, 2001, 47(5): 1077-1087. doi: 10.1002/aic.690470514 [5] KIM H J, KIM S J, HYEON S, et al. Application of desalination membranes to nuclide (Cs, Sr, and Co) separation[J]. ACS Omega, 2020, 5(32): 20261-20269. doi: 10.1021/acsomega.0c02106 [6] SASAKI T, OKABE J, HENMI M, et al. Cesium (Cs) and strontium (Sr) removal as model materials in radioactive water by advanced reverse osmosis membrane[J]. Desalination and Water Treatment, 2013, 51(7-9): 1672-1677. doi: 10.1080/19443994.2012.704696 [7] OZAKI H, LI H F. Rejection of organic compounds by ultra-low pressure reverse osmosis membrane[J]. Water Research, 2002, 36(1): 123-130. doi: 10.1016/S0043-1354(01)00197-X [8] CHEN D, ZHAO X, LI F Z. Treatment of low level radioactive wastewater by means of NF process[J]. Nuclear Engineering and Design, 2014, 278: 249-254. doi: 10.1016/j.nucengdes.2014.08.001 [9] MA B, OH S, SHIN W S, et al. Removal of Co2+, Sr2+ and Cs+ from aqueous solution by phosphate-modified montmorillonite (PMM)[J]. Desalination, 2011, 276(1-3): 336-346. doi: 10.1016/j.desal.2011.03.072 [10] LEE B S. Nuclide separation modeling through reverse osmosis membranes in radioactive liquid waste[J]. Nuclear Engineering and Technology, 2015, 47(7): 859-866. doi: 10.1016/j.net.2015.08.001 [11] 何利斌,徐文露,顾平,等. 单级超低压反渗透膜工艺处理模拟放射性锶废水[J]. 中国给水排水,2021, 37(9): 105-109. doi: 10.19853/j.zgjsps.1000-4602.2021.09.017 [12] 李志超. 反渗透技术处理模拟含碘、铯、锶放射性废水的研究[D]. 天津: 天津大学, 2018: 39-54. [13] OZAKI H, SHARMA K, SAKTAYWIN W. Performance of an ultra-low-pressure reverse osmosis membrane (ULPROM) for separating heavy metal: effects of interference parameters[J]. Desalination, 2002, 144(1-3): 287-294. doi: 10.1016/S0011-9164(02)00329-6 [14] FIRDAOUS L, QUÉMÉNEUR F, SCHLUMPF J P, et al. Modification of the ionic composition of salt solutions by electrodialysis[J]. Desalination, 2004, 167: 397-402. doi: 10.1016/j.desal.2004.06.153 [15] ZHANG L, WEI J Y, ZHAO X, et al. Removal of strontium(II) and cobalt(II) from acidic solution by manganese antimonate[J]. Chemical Engineering Journal, 2016, 302: 733-743. doi: 10.1016/j.cej.2016.05.040 [16] DING S Y, YANG Y, HUANG H O, et al. Effects of feed solution chemistry on low pressure reverse osmosis filtration of cesium and strontium[J]. Journal of Hazardous Materials, 2015, 294: 27-34. doi: 10.1016/j.jhazmat.2015.03.056 [17] CHEN D, LI F Z, ZHAO X, et al. The influence of salts on the reverse osmosis performance treating simulated boron-containing low level radioactive wastewater[J]. Journal of Chemical Technology & Biotechnology, 2018, 93(12): 3607-3612. [18] CHILDRESS A E, ELIMELECH M. Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes[J]. Journal of Membrane Science, 1996, 119(2): 253-268. doi: 10.1016/0376-7388(96)00127-5 [19] SHIM Y, LEE H J, LEE S, et al. Effects of natural organic matter and ionic species on membrane surface charge[J]. Environmental Science & Technology, 2002, 36(17): 3864-3871. [20] LI Q L, ELIMELECH M. Organic fouling and chemical cleaning of nanofiltration membranes: measurements and mechanisms[J]. Environmental Science & Technology, 2004, 38(17): 4683-4693. [21] DING S Y, YANG Y, LI C, et al. The effects of organic fouling on the removal of radionuclides by reverse osmosis membranes[J]. Water Research, 2016, 95: 174-184. doi: 10.1016/j.watres.2016.03.028 -





 下载:
下载: