Study on the Steady γ-Radiolysis of Ammonia Solution
-
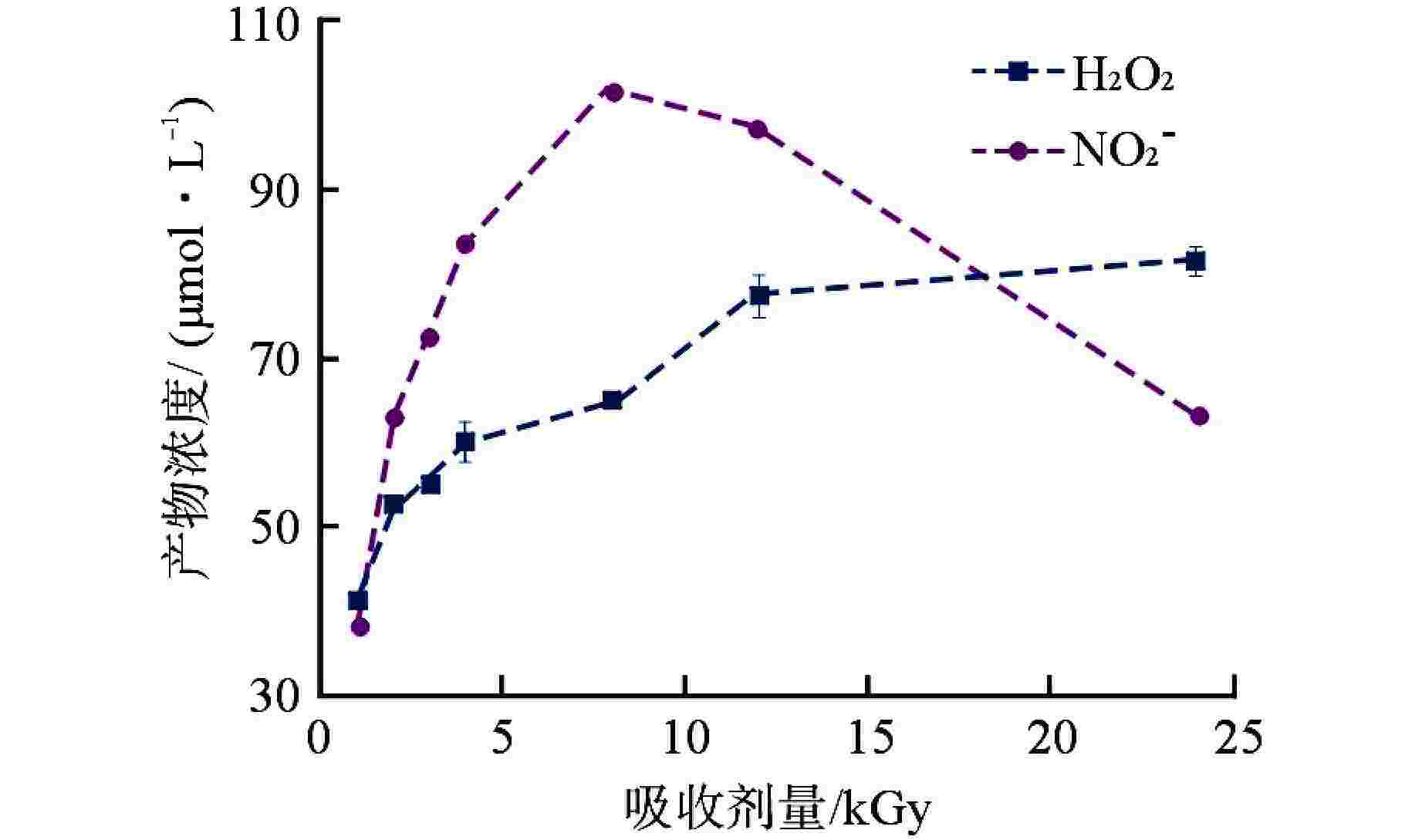
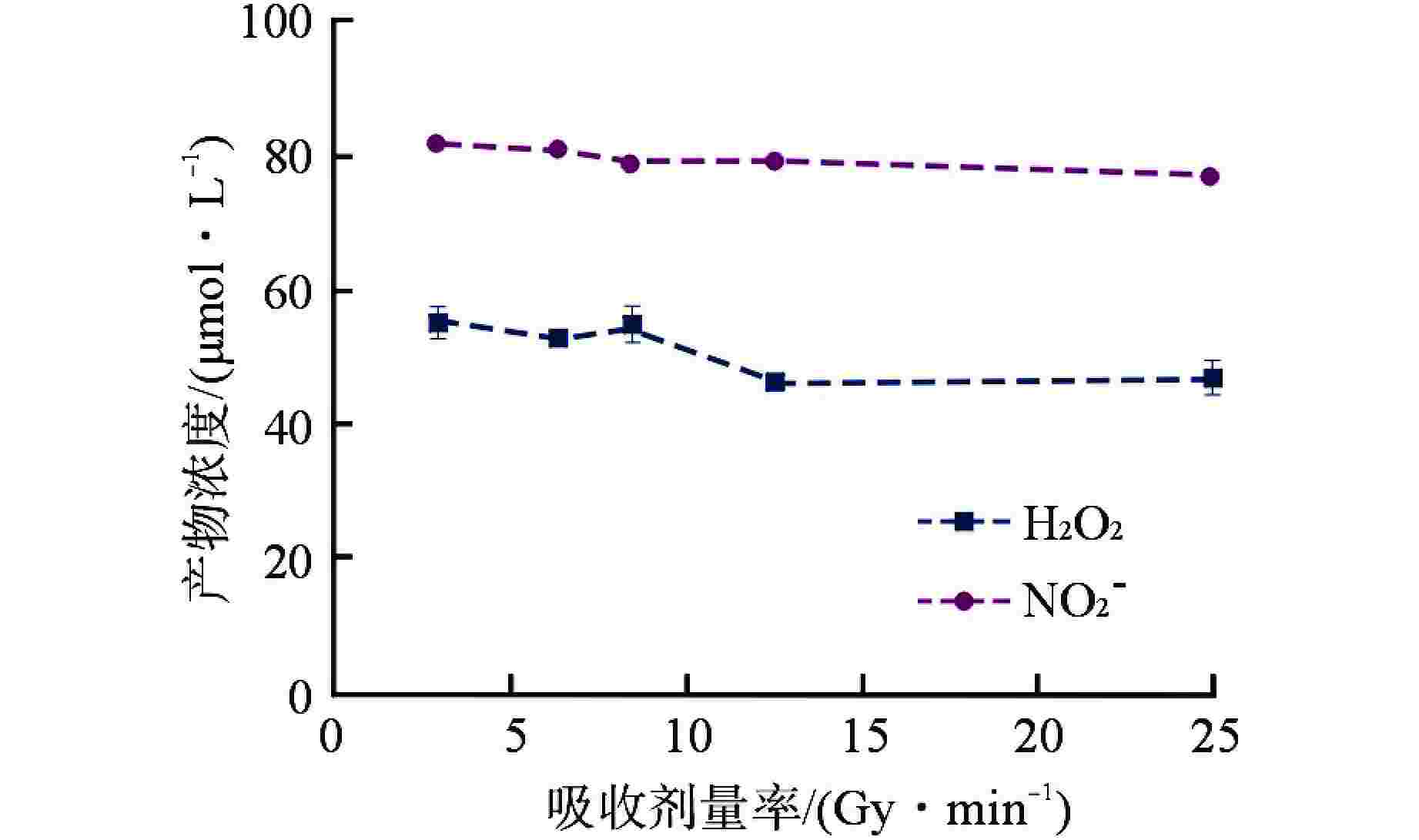
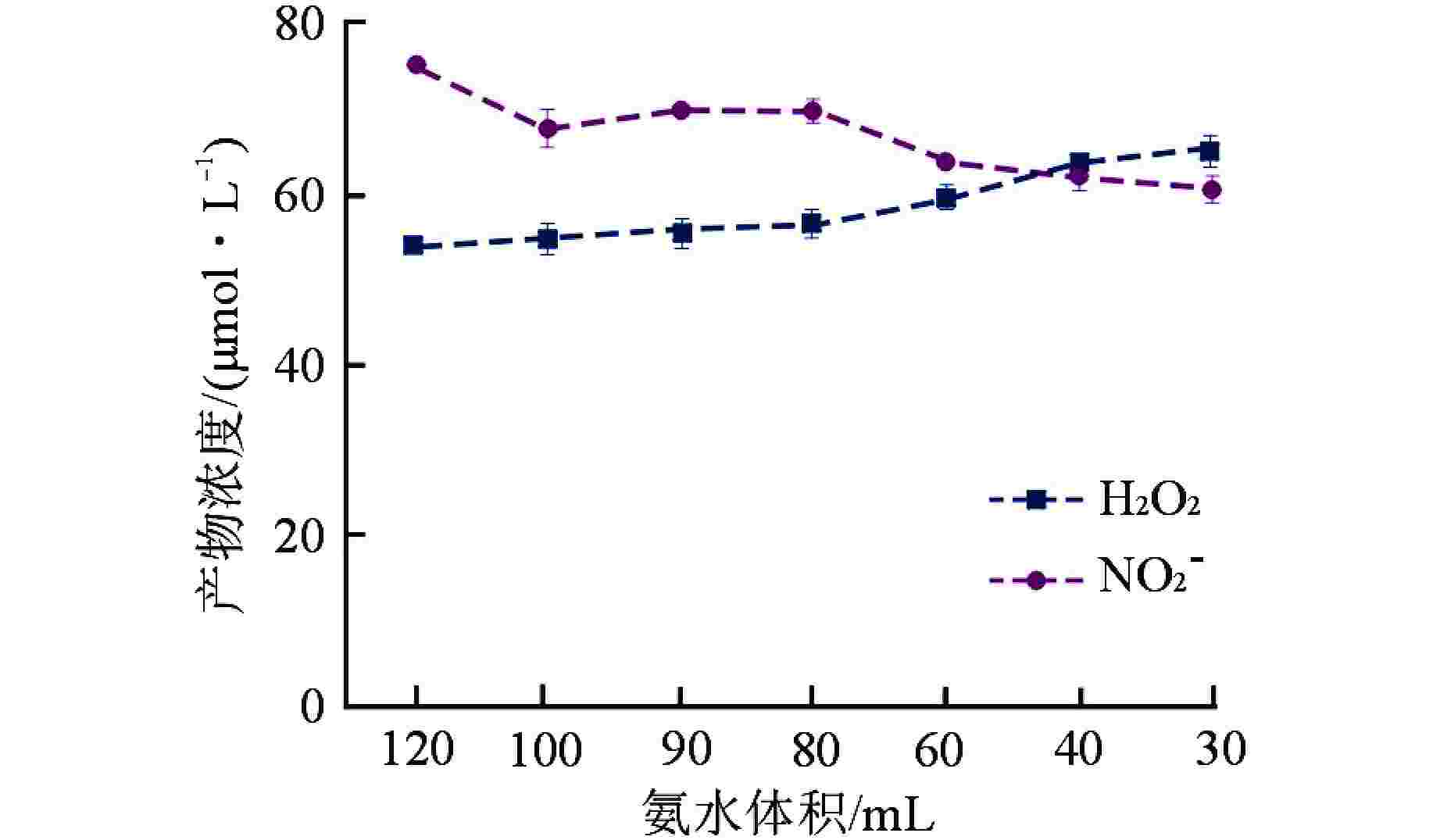
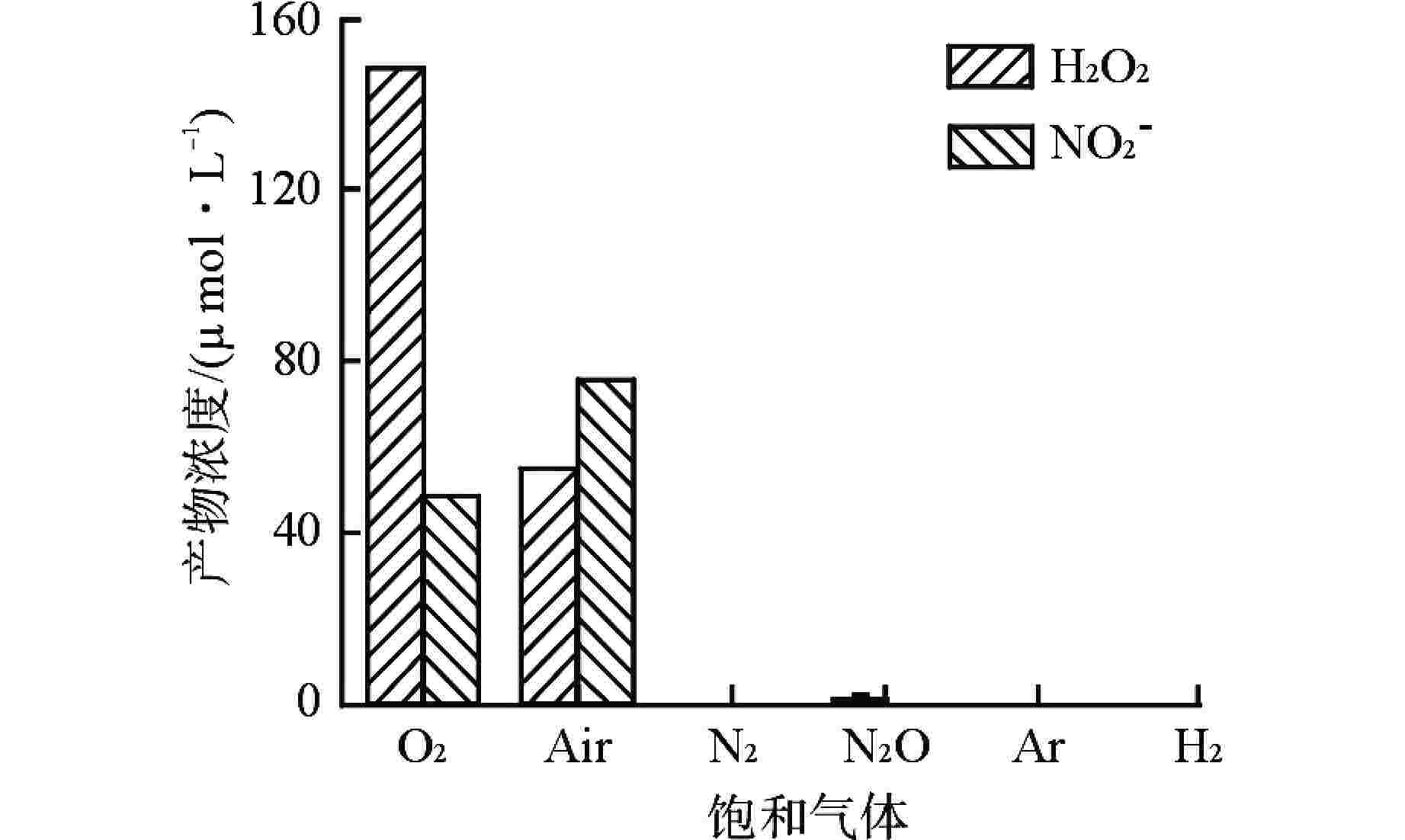
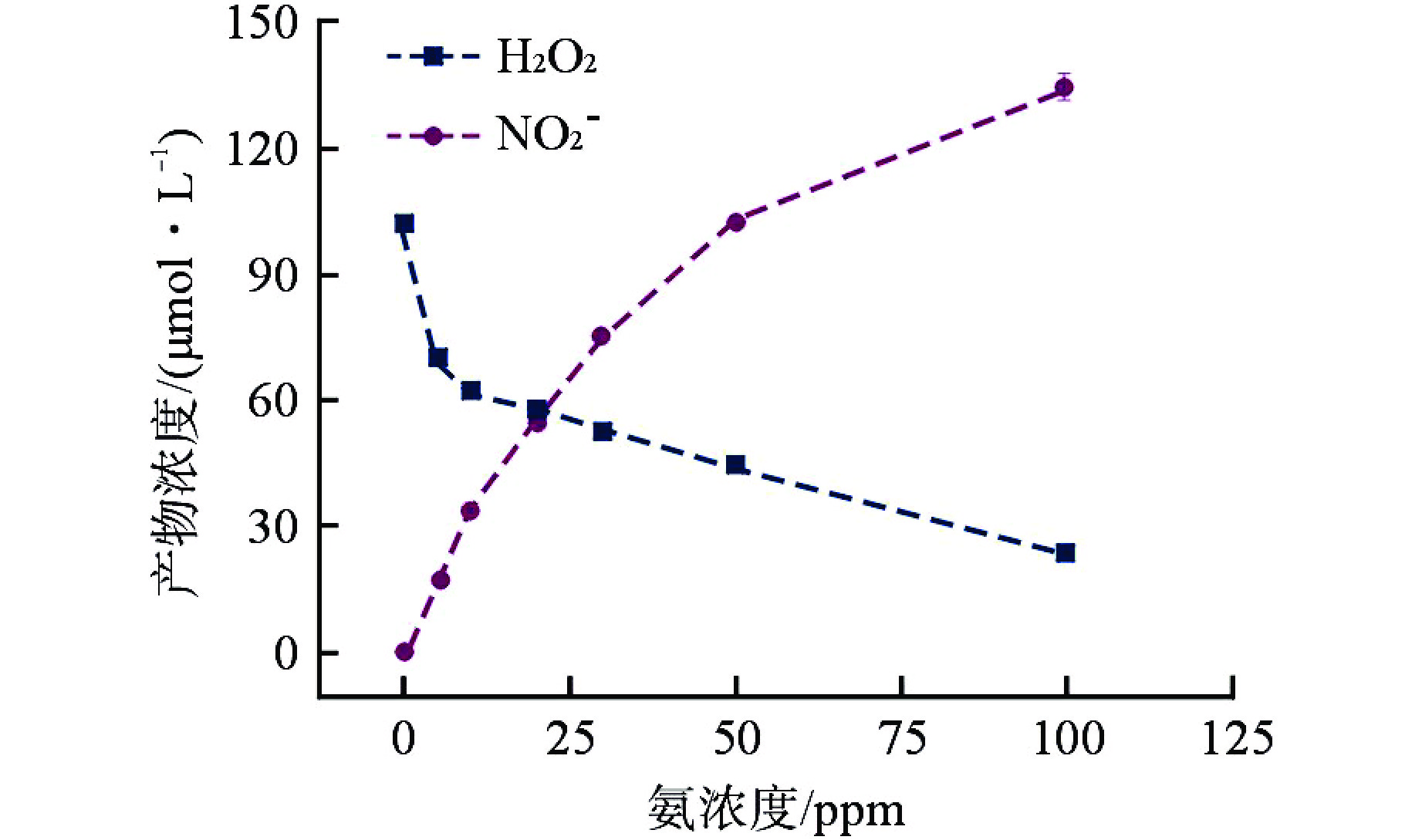
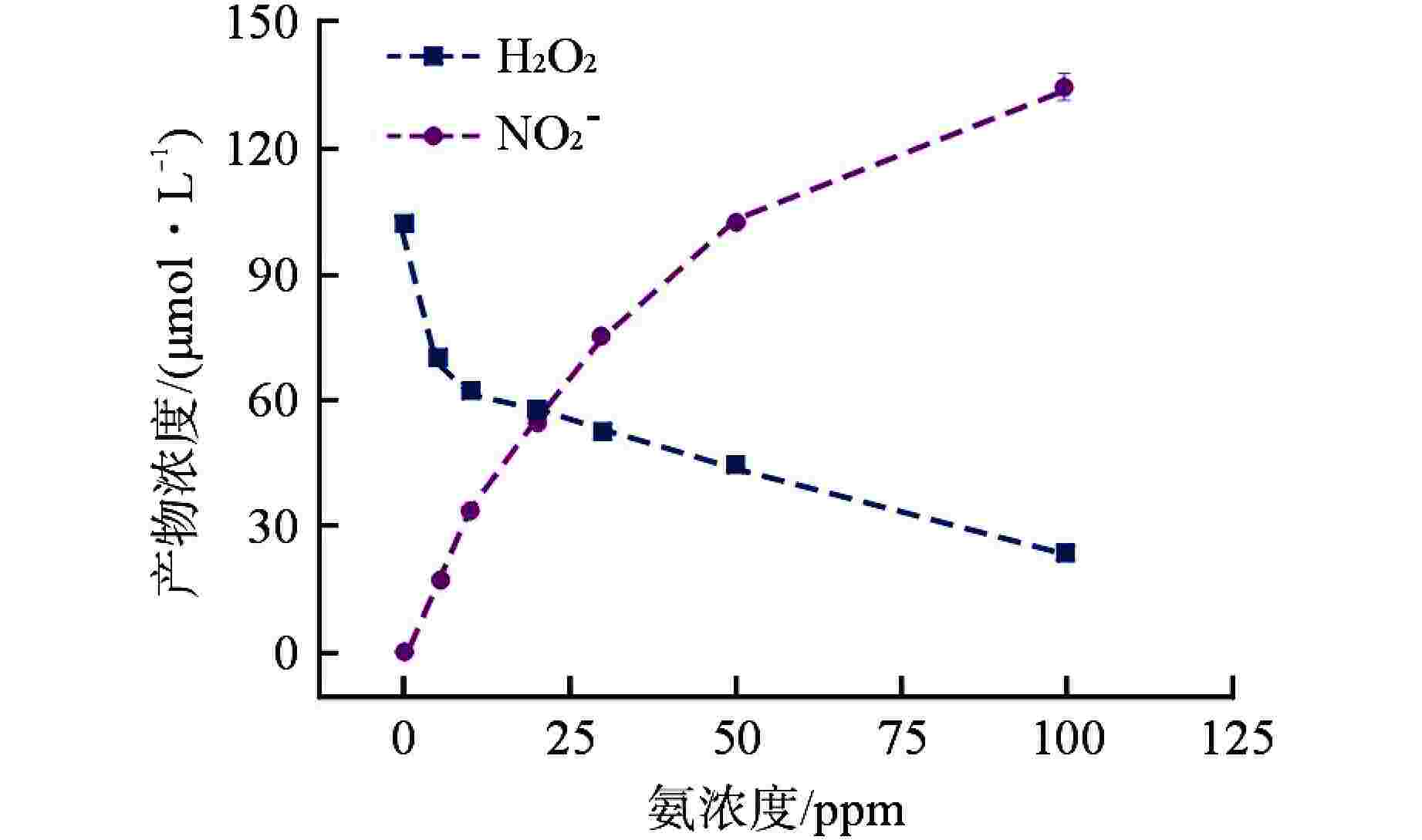
摘要: 含氨冷却剂被应用于压水堆中,可以清除冷却剂辐射分解产生的O2、H2O2等氧化性产物,从而减轻结构材料腐蚀。本文研究了氨水在γ辐射场中的辐射分解行为,考察氨浓度、辐射吸收剂量和吸收剂量率、气液体积比和不同饱和气体对氨水辐射分解行为的影响,重点关注辐射分解产物H2O2和NO2−的浓度变化。结果表明,随着体系中氨浓度的增加,H2O2的浓度受到明显抑制,NO2−的浓度则呈现出上升趋势;吸收剂量的增加使得H2O2浓度明显升高,NO2−的浓度则在吸收剂量为8 kGy时达到最大(> 100 μmol/L),而后降低;吸收剂量率的差异(2.78~25 Gy/min)并未导致H2O2和NO2−浓度产生明显变化;氨水中的O2是NO2−生成的关键,但O2过多会促进NO2−氧化为NO3−从而降低NO2−浓度,此外O2的存在促进了H2O2的生成。本文研究结果可为后续含氨冷却剂体系的优化提供参考。Abstract: Ammonia is applied in the coolant to eliminate the O2 and H2O2 in the PWR, thus mitigating the corrosion of structural materials. The present work studied the γ-radiolysis of ammonia solution under different conditions including ammonia concentration, absorbed dose, absorbed dose rate, gas-liquid volume ratio, and different saturated gases. The results show that ammonia significantly inhibits H2O2 because of the consumption of H2O2 and suppression of its precursors. The concentration of NO2− rises with the ammonia concentration . Ammonia is continuously consumed as irradiation progresses, while the concentration of H2O2 increases significantly with the absorbed dose. The NO2− reaches a peak maximum (> 100 μmol/L) when the absorbed dose is 8 kGy. However, NO2− has a drop when the absorbed dose grows further, due to the fact O2 oxidizes NO2− into NO3−. The concentrations of H2O2 and NO2− are not obviously affected within the absorbed dose rate range (2.78~25 Gy/min). The presence of O2 is critical to the formation of NO2−, though excessive O2 in the system could lead to the oxidization of NO2− by increasing the oxidizing species. Besides, the oxygen dissolved in aqueous ammonia promotes the production of H2O2. This work is expected to provide a helpful reference for the optimization of the ammonia-containing coolant system.
-
Key words:
- Ammonia /
- Coolant /
- Radiolysis /
- Reactor /
- Water chemistry
-
-
[1] WREN J C. Steady-state radiolysis: effects of dissolved additives[M]. United States: ACS Publications, 2010: 271-295. [2] UCHIDA S, KATSUMURA Y. Water chemistry technology – one of the key technologies for safe and reliable nuclear power plant operation[J]. Journal of Nuclear Science and Technology, 2013, 50(4): 346-362. doi: 10.1080/00223131.2013.773171 [3] ELLIOT A, BARTELS D. The reaction set, rate constants and g-values for the simulation of the radiolysis of light water over the range 20℃ to 350℃ based on information available in 2008: AECL-153-127160-450-001[R]. Canada: Atomic Energy of Canada Limited, 2009 [4] DAUB K, ZHANG X, NOËL J J, et al. Effects of γ-radiation versus H2O2 on carbon steel corrosion[J]. Electrochimica Acta, 2010, 55(8): 2767-2776. doi: 10.1016/j.electacta.2009.12.028 [5] BULANOV A V, KOLESOV B I, LUKASHENKO M L, et al. Radiolysis of ammonia in the first-loop coolant of reactors in floating power-generating units[J]. Atomic Energy, 2000, 88(5): 368-372. doi: 10.1007/BF02680531 [6] HICKEL B, SEHESTED K. Reaction of hydroxyl radicals with ammonia in liquid water at elevated temperatures[J]. International Journal of Radiation Applications and Instrumentation. Part C. Radiation Physics and Chemistry, 1992, 39(4): 355-357. doi: 10.1016/1359-0197(92)90244-A [7] PAGSBERG P B. Investigation of the NH2 radical produced by pulse radiolysis of ammonia in aqueous solution: RISØ-256[R]. Denmark: Danish Atomic Energy Commission, 1972. [8] NETA P, MARUTHAMUTHU P, CARTON P M, et al. Formation and reactivity of the amino radical[J]. The Journal of Physical Chemistry, 1978, 82(17): 1875-1878. doi: 10.1021/j100506a004 [9] LUZAKOV A V, BULANOV A V, VERKHOVSKAYA A O, et al. Radiation-chemical removal of corrosion hydrogen from VVER first-loop coolant[J]. Atomic Energy, 2008, 105(6): 402-407. doi: 10.1007/s10512-009-9115-4 [10] RIGG T, SCHOLES G, WEISS J. 580. Chemical actions of ionising radiations in solutions. Part X. The action of X-rays on ammonia in aqueous solution[J]. Journal of the Chemical Society (Resumed), 1952: 3034-3038. [11] DWIBEDY P, KISHORE K, DEY G R, et al. Nitrite formation in the radiolysis of aerated aqueous solutions of ammonia[J]. Radiation Physics and Chemistry, 1996, 48(6): 743-747. doi: 10.1016/S0969-806X(96)00037-0 [12] GHORMLEY J A. Warming curves for the condensed product of dissociated water vapor and for hydrogen peroxide glass[J]. Journal of the American Chemical Society, 1957, 79(8): 1862-1865. doi: 10.1021/ja01565a025 [13] SHINN M B. Colorimetric method for determination of nitrate[J]. Industrial & Engineering Chemistry Analytical Edition, 1941, 13(1): 33-35. [14] LIU Z, FANG Z, WANG L, et al. Alpha radiolysis of nitric acid aqueous solution irradiated by 238Pu source[J]. Nuclear Science and Techniques, 2017, 28(4): 54. doi: 10.1007/s41365-017-0200-4 [15] BUXTON G V, GREENSTOCK C L, HELMAN W P, et al. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in Aqueous Solution[J]. Journal of Physical and Chemical Reference Data, 1988, 17(2): 513-886. doi: 10.1063/1.555805 [16] 彭静, 郝燕, 魏根栓, 等. 辐射化学基础教程[M]. 北京: 北京大学出版社, 2015: 186-187. [17] STEINBERG M. Irradiation of NH3-H2O solutions for formation of hydrazine: BNL-613(T-183)[R]. United States: Brookhaven National Laboratory, 1960 [18] BUXTON G V, DAINTON F S. Radical and molecular yields in the γ-radiolysis of water. II. The potassium iodide-nitrous oxide system in the pH range 0 to 14[J]. Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences, 1965, 287(1411): 427-443. [19] SCHULER R H, PATTERSON L K, JANATA E. Yield for the scavenging of hydroxyl radicals in the radiolysis of nitrous oxide-saturated aqueous solutions[J]. Journal of Physical Chemistry, 1980, 84(16): 2088-2089. doi: 10.1021/j100453a020 -





 下载:
下载: