Optimization of Oxygen Control Strategy for Corrosion Mitigation in Lead-Bismuth Cooled Fast Reactors
-
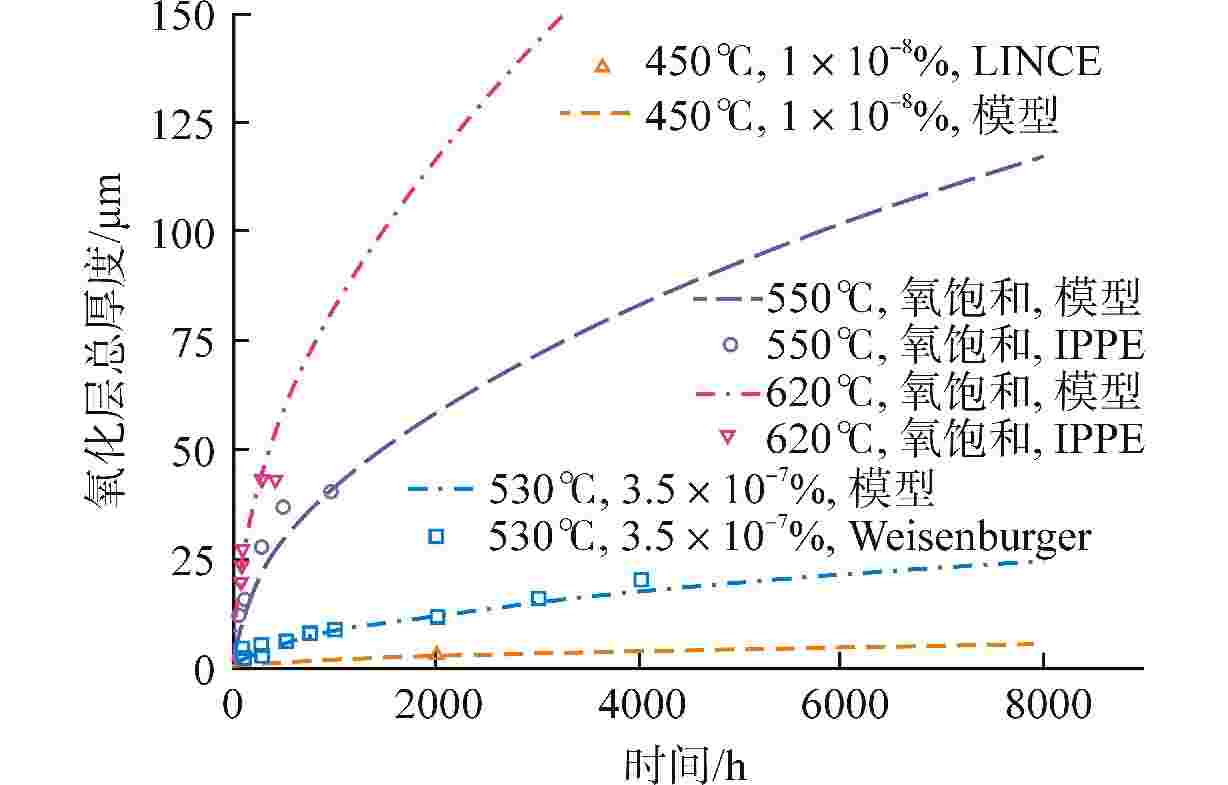
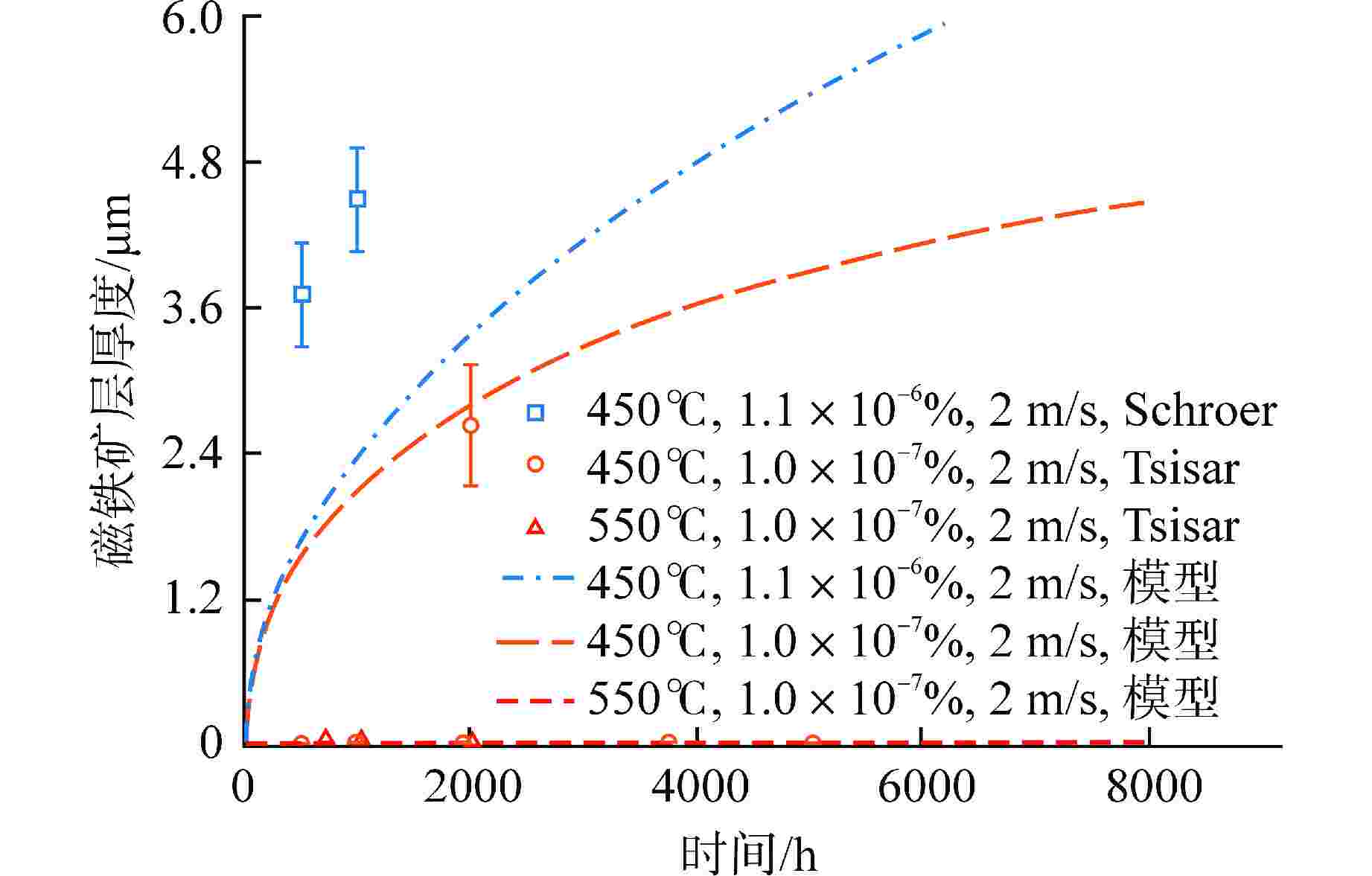
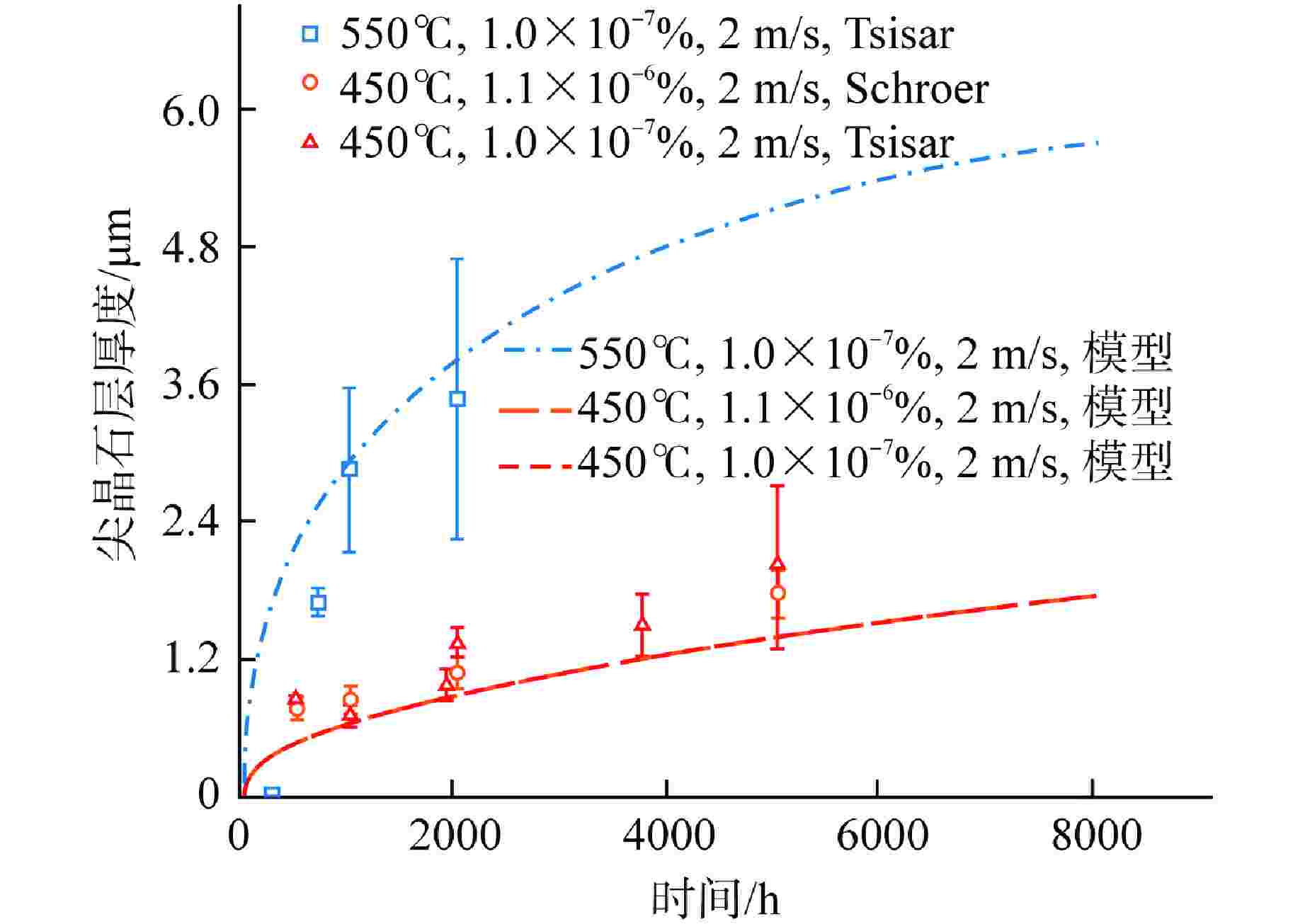
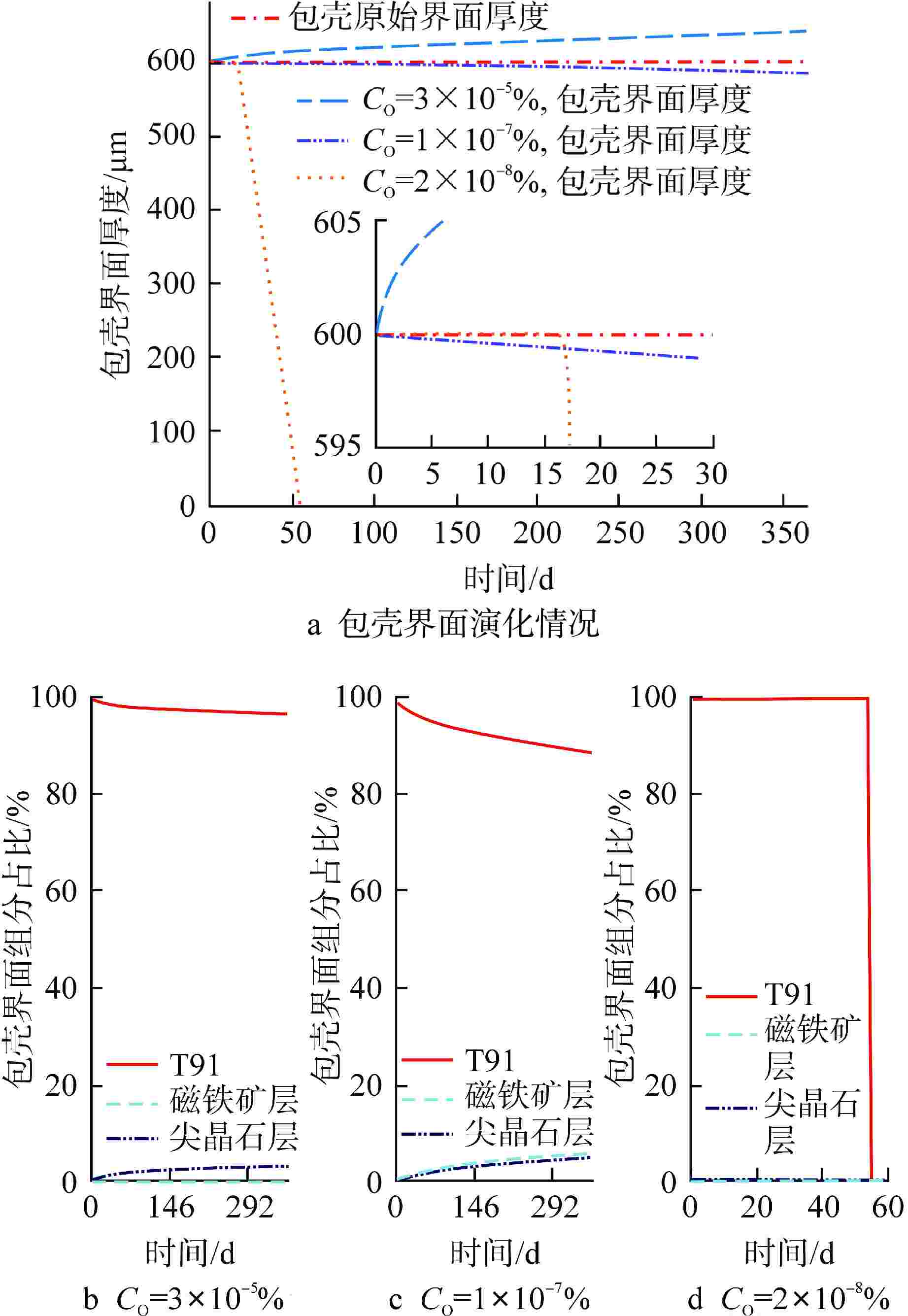
摘要: 为获得铅铋快堆燃料包壳腐蚀缓解的最优氧含量控制策略,本研究通过构建T91氧化/腐蚀模型,分析了燃料元件包壳界面的演化规律。在此基础上,以氧化层厚度为优化问题的约束条件,采用鲸鱼优化算法(WOA)对氧含量控制策略进行了寻优分析,得到“低-中-高-低”循环波动氧含量控制策略。此外,本研究对固定氧主导条件与优化氧含量控制策略下的燃料元件表面氧化层分布进行了模拟对比。研究结果表明,在优化氧含量控制策略下,燃料元件包壳未触发溶解腐蚀,且表面氧化层的整体厚度较固定氧主导工况有显著减少,其中磁铁矿层平均厚度同比下降95.6%;尖晶石层平均厚度下降44.2%,本文所构建的最优氧含量控制策略可为铅铋快堆包壳腐蚀缓解提供参考。Abstract: To obtain the optimal oxygen content control strategy for mitigating corrosion of fuel cladding in lead-bismuth fast reactors (LBFRs), this study constructed a T91 oxidation/corrosion model to analyze the evolution of the fuel element cladding interface. On this basis, taking the thickness of oxide layer as the constraint condition of the optimization problem, the whale optimization algorithm (WOA) was used to optimize the oxygen content control strategy, and the "low-medium-high-low" cyclic fluctuation oxygen content control strategy was obtained. Furthermore, this study simulated and compared the distribution of the oxide layer on the surface of fuel elements under fixed oxygen-dominated condition and optimized oxygen control strategy. The results indicated that under the optimized oxygen control strategy, the fuel element cladding did not trigger dissolution corrosion, and the overall thickness of the surface oxide layer was significantly reduced compared to the fixed oxygen-dominated condition, with an average thickness reduction of 95.6% for the magnetite layer and 44.2% for the spinel layer. The optimal oxygen content control strategy constructed in this paper can provide a reference for mitigating corrosion of cladding in lead-bismuth fast reactors.
-
表 1 典型铅基快堆设计参数
Table 1. Design Parameters of Typical Lead-based Fast Reactor
参数名 参数值 堆芯质量流量/(kg·s−1) 12238 堆芯热功率/MW 1500 冷却剂最大流速/(m·s−1) 2 冷却剂入口温度/K 673 堆芯活性段高度/m 1.2 包壳外径/mm 10.6 包壳内径/mm 9.4 -
[1] SMITH C F, CINOTTI L. Lead-cooled fast reactors (LFRs)[M]//PIORO I L. Handbook of Generation IV Nuclear Reactors. 2nd ed. Cambridge: Elsevier, 2023: 195-230. [2] OECD/NEA. Handbook on lead-bismuth eutectic alloy and lead properties, materials compatibility, thermal-hydraulics and technologies[M]. Paris: OECD Publishing, 2015: 17-23. [3] BALLINGER R G, LIM J. An overview of corrosion issues for the design and operation of high-temperature lead- and lead-bismuth-cooled reactor systems[J]. Nuclear Technology, 2004, 147(3): 418-435. doi: 10.13182/NT04-A3540 [4] FENG W P, ZHANG X, CAO L K, et al. Development of oxygen/corrosion product mass transfer model and oxidation-corrosion model in the lead-alloy cooled reactor core[J]. Corrosion Science, 2021, 190: 109708. doi: 10.1016/j.corsci.2021.109708 [5] KIESER M, MUSCHER H, WEISENBURGER A, et al. Liquid metal corrosion/erosion investigations of structure materials in lead cooled systems: part 1[J]. Journal of Nuclear Materials, 2009, 392(3): 405-412. doi: 10.1016/j.jnucmat.2008.12.327 [6] KONDO M, MUROGA T, SAGARA A, et al. Flow accelerated corrosion and erosion–corrosion of RAFM steel in liquid breeders[J]. Fusion Engineering and Design, 2011, 86(9-11): 2500-2503. doi: 10.1016/j.fusengdes.2011.01.108 [7] KONDO M, TAKAHASHI M. Corrosion resistance of Si- and Al-rich steels in flowing lead–bismuth[J]. Journal of Nuclear Materials, 2006, 356(1-3): 203-212. doi: 10.1016/j.jnucmat.2006.05.019 [8] LI C, LIU Y J, ZHANG F F, et al. Erosion-corrosion of 304N austenitic steels in liquid Pb-Bi flow perpendicular to steel surface[J]. Materials Characterization, 2021, 175: 111054. doi: 10.1016/j.matchar.2021.111054 [9] LI N. Active control of oxygen in molten lead–bismuth eutectic systems to prevent steel corrosion and coolant contamination[J]. Journal of Nuclear Materials, 2002, 300(1): 73-81. doi: 10.1016/S0022-3115(01)00713-9 [10] MARINO A, BUCKINGHAM S, GLADINEZ K, et al. Numerical modeling of iron-based corrosion product oxides mass transport in the MYRRHA reactor during normal operation[J]. Nuclear Engineering and Design, 2018, 338: 199-208. doi: 10.1016/j.nucengdes.2018.08.008 [11] MARTINELLI L, BALBAUD-CÉLÉRIER F. Modelling of the oxide scale formation on Fe-Cr steel during exposure in liquid lead-bismuth eutectic in the 450-600℃ temperature range[J]. Materials and Corrosion, 2011, 62(6): 531-542. doi: 10.1002/maco.201005871 [12] MARTINELLI L, BALBAUD-CÉLÉRIER F, PICARD G, et al. Oxidation mechanism of a Fe-9Cr-1Mo steel by liquid Pb–Bi eutectic alloy (Part III)[J]. Corrosion Science, 2008, 50(9): 2549-2559. doi: 10.1016/j.corsci.2008.06.049 [13] MARTINELLI L, BALBAUD-CÉLÉRIER F, TERLAIN A, et al. Oxidation mechanism of an Fe–9Cr–1Mo steel by liquid Pb–Bi eutectic alloy at 470℃ (Part II)[J]. Corrosion Science, 2008, 50(9): 2537-2548. doi: 10.1016/j.corsci.2008.06.051 [14] MARTINELLI L, BALBAUD-CÉLÉRIER F, TERLAIN A, et al. Oxidation mechanism of a Fe–9Cr–1Mo steel by liquid Pb–Bi eutectic alloy (Part I)[J]. Corrosion Science, 2008, 50(9): 2523-2536. doi: 10.1016/j.corsci.2008.06.050 [15] MARTINELLI L, DUFRENOY T, JAAKOU K, et al. High temperature oxidation of Fe–9Cr–1Mo steel in stagnant liquid lead–bismuth at several temperatures and for different lead contents in the liquid alloy[J]. Journal of Nuclear Materials, 2008, 376(3): 282-288. doi: 10.1016/j.jnucmat.2008.02.006 [16] MARTINELLI L, GINESTAR K, BOTTON V, et al. Corrosion of T91 and pure iron in flowing and static Pb-Bi alloy between 450℃ and 540℃: experiments, modelling and mechanism[J]. Corrosion Science, 2020, 176: 108897. doi: 10.1016/j.corsci.2020.108897 [17] SCHROER C, WEDEMEYER O, SKRYPNIK A, et al. Corrosion kinetics of Steel T91 in flowing oxygen-containing lead–bismuth eutectic at 450℃[J]. Journal of Nuclear Materials, 2012, 431(1-3): 105-112. doi: 10.1016/j.jnucmat.2011.11.014 [18] BARBIER F, BENAMATI G, FAZIO C, et al. Compatibility tests of steels in flowing liquid lead–bismuth[J]. Journal of Nuclear Materials, 2001, 295(2-3): 149-156. doi: 10.1016/S0022-3115(01)00570-0 [19] SCHROER C, VOß Z, WEDEMEYER O, et al. Oxidation of steel T91 in flowing lead–bismuth eutectic (LBE) at 550℃[J]. Journal of Nuclear Materials, 2006, 356(1-3): 189-197. doi: 10.1016/j.jnucmat.2006.05.009 [20] ZHANG J S, LI N. Analysis on liquid metal corrosion–oxidation interactions[J]. Corrosion Science, 2007, 49(11): 4154-4184. doi: 10.1016/j.corsci.2007.05.012 [21] BALBAUD-CÉLÉRIER F, BARBIER F. Investigation of models to predict the corrosion of steels in flowing liquid lead alloys[J]. Journal of Nuclear Materials, 2001, 289(3): 227-242. doi: 10.1016/S0022-3115(01)00431-7 [22] AERTS A, GAVRILOV S, MANFREDI G, et al. Oxygen–iron interaction in liquid lead–bismuth eutectic alloy[J]. Physical Chemistry Chemical Physics, 2016, 18(29): 19526-19530. doi: 10.1039/C6CP01561A [23] ZHANG J S, LI N, CHEN Y T, et al. Corrosion behaviors of US steels in flowing lead–bismuth eutectic (LBE)[J]. Journal of Nuclear Materials, 2005, 336(1): 1-10. doi: 10.1016/j.jnucmat.2004.08.002 [24] ZHANG J, HOSEMANN P, MALOY S. Models of liquid metal corrosion[J]. Journal of Nuclear Materials, 2010, 404(1): 82-96. doi: 10.1016/j.jnucmat.2010.05.024 [25] SILVERMAN D C. Technical note: on estimating conditions for simulating velocity-sensitive corrosion in the rotating cylinder electrode[J]. Corrosion, 1999, 55(12): 1115-1118. doi: 10.5006/1.3283948 [26] GU Z X, ZHANG Q X, GU Y, et al. Verification of a self-developed CFD-based multi-physics coupled code MPC-LBE for LBE-cooled reactor[J]. Nuclear Science and Techniques, 2021, 32(5): 52. doi: 10.1007/s41365-021-00887-x [27] LU D S, WANG C, WANG C L, et al. Numerical simulation of corrosion phenomena in oxygen-controlled environment for a horizontal lead-bismuth reactor core[J]. Journal of Nuclear Materials, 2023, 574: 154195. doi: 10.1016/j.jnucmat.2022.154195 [28] TSISAR V, SCHROER C, WEDEMEYER O, et al. Characterization of corrosion phenomena and kinetics on T91 ferritic/martensitic steel exposed at 450 and 550℃ to flowing Pb-Bi eutectic with 10−7 mass% dissolved oxygen[J]. Journal of Nuclear Materials, 2017, 494: 422-438. doi: 10.1016/j.jnucmat.2017.07.031 [29] ALEMBERTI A, CARLSSON J, MALAMBU E, et al. European lead fast reactor—ELSY[J]. Nuclear Engineering and Design, 2011, 241(9): 3470-3480. doi: 10.1016/j.nucengdes.2011.03.029 [30] MIRJALILI S, LEWIS A. The whale optimization algorithm[J]. Advances in Engineering Software, 2016, 95: 51-67. doi: 10.1016/j.advengsoft.2016.01.008 -






 下载:
下载: