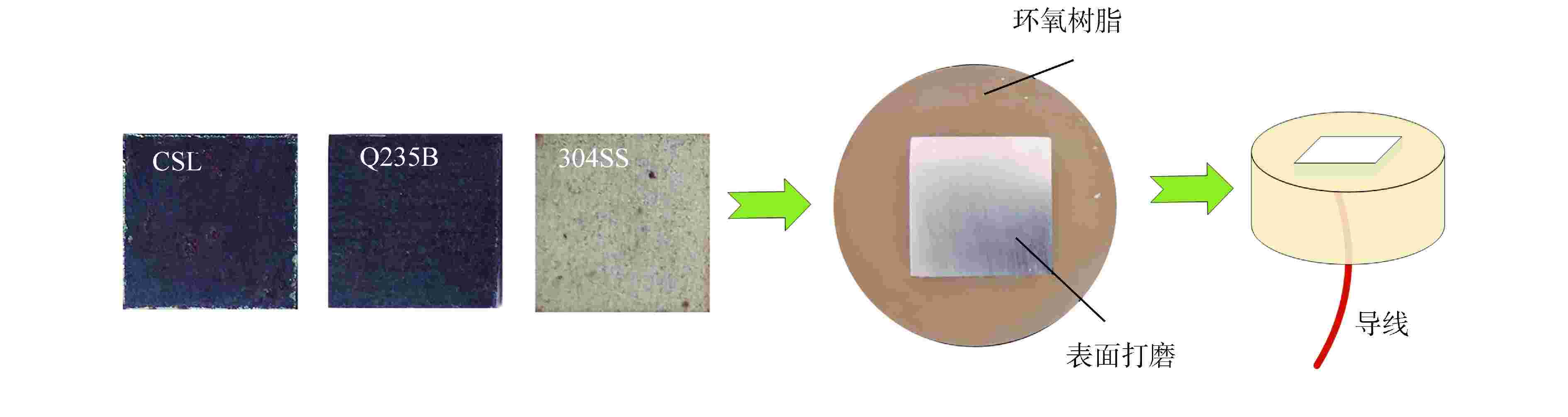
Reasearch on Corrosion Resistance of Containment Steel Liner in Simulated Concrete Pore Solution
-
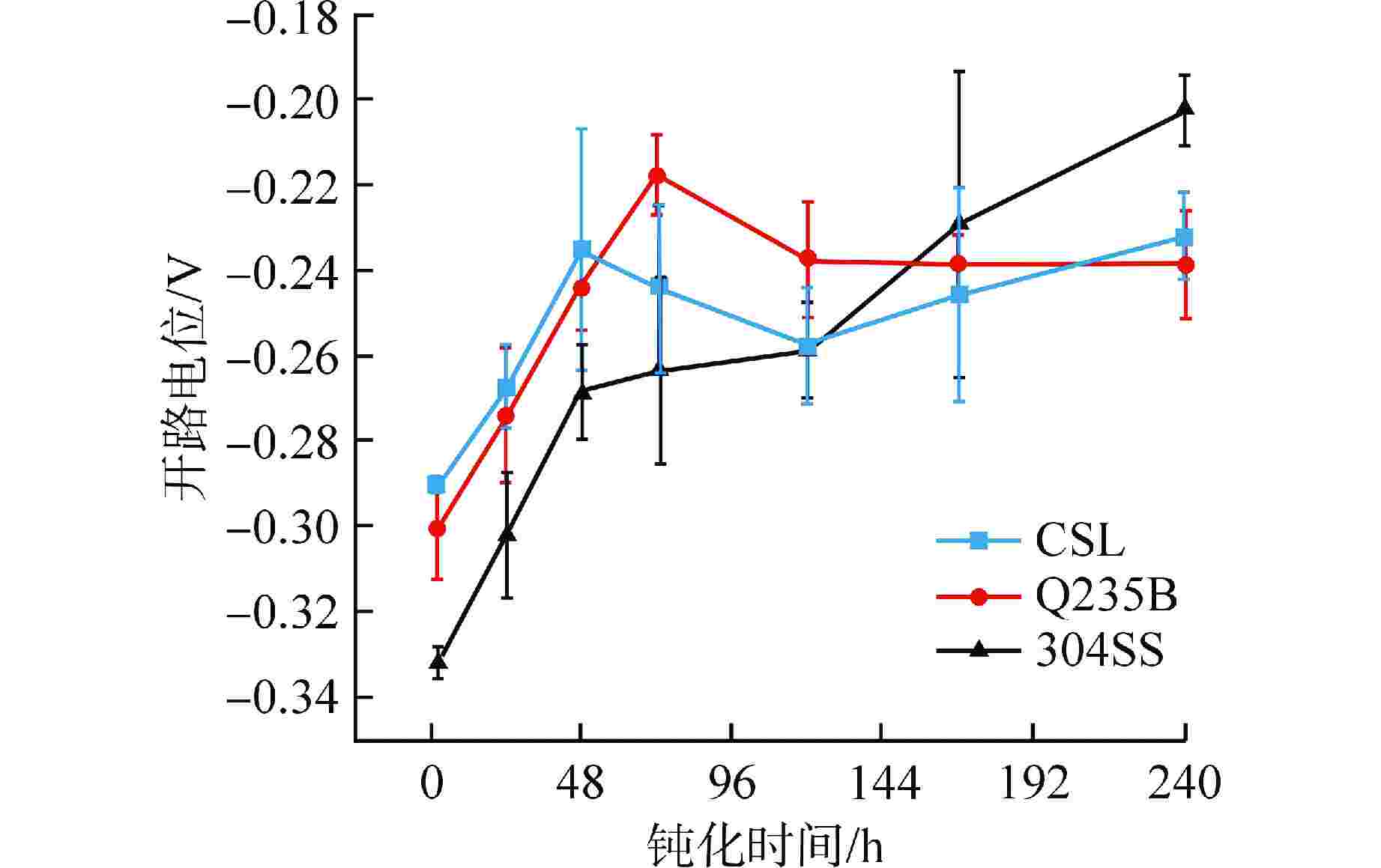
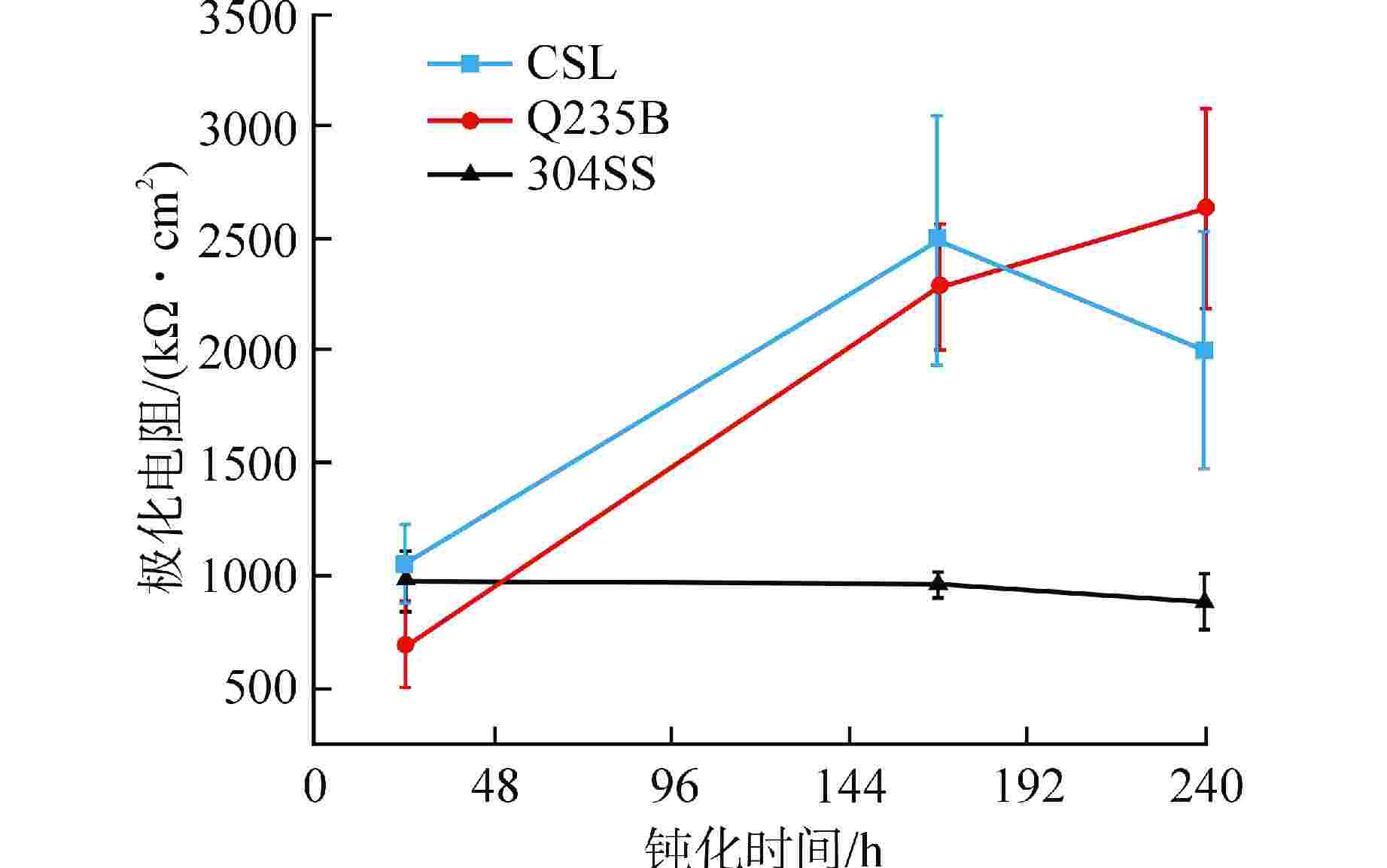
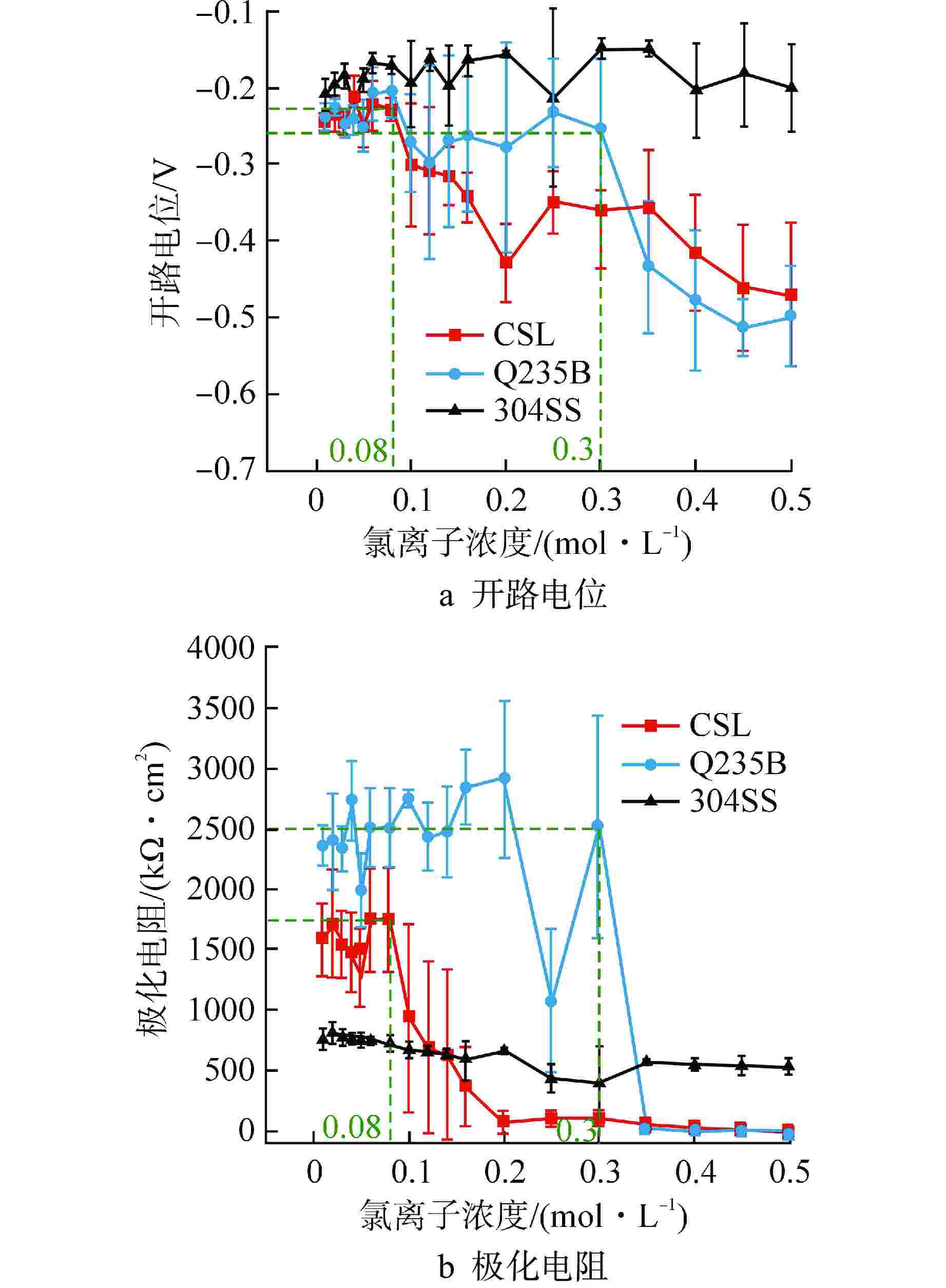
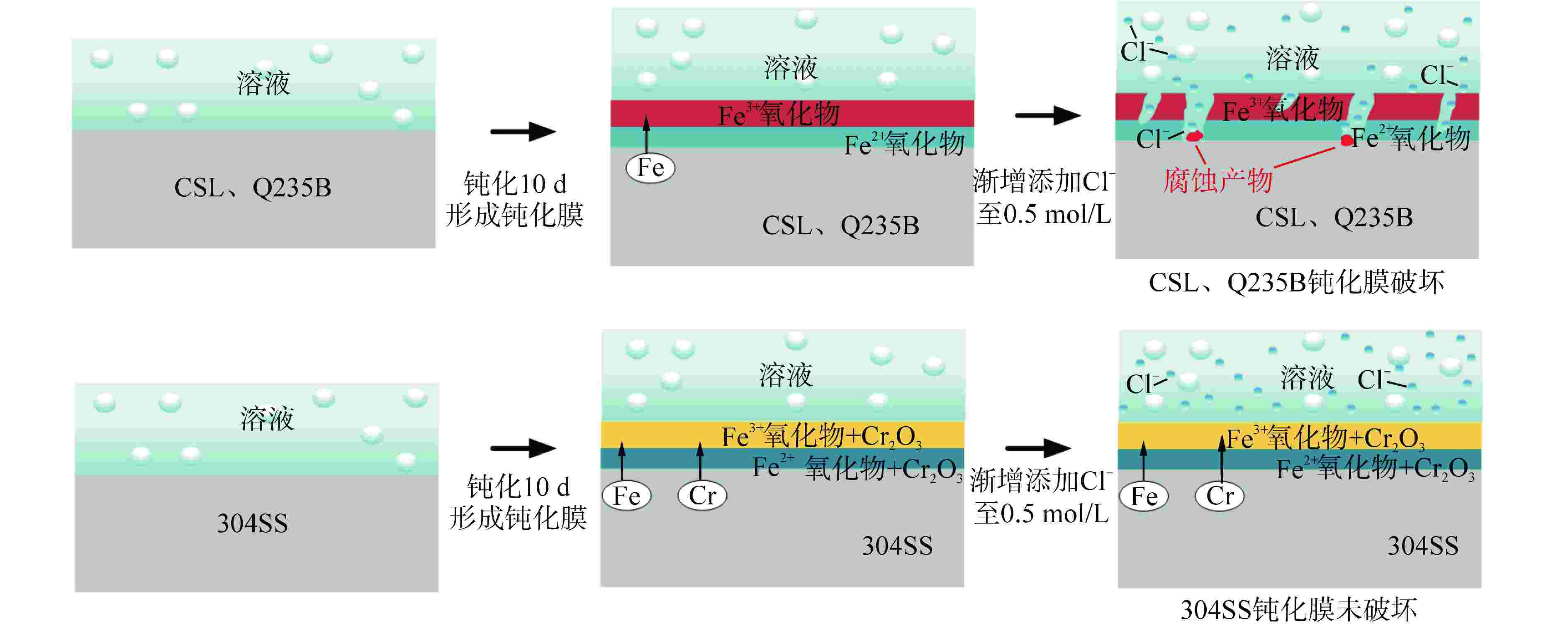
摘要: 针对核电厂安全壳钢衬里专用P265GH低合金钢(CSL),采用电化学和X射线光电子能谱(XPS)分析方法探究其在饱和Ca(OH)2溶液中的钝化和破钝效应,并与Q235B低碳钢(Q235B)和304不锈钢(304SS)耐腐蚀性能进行对比分析。结果表明:与Q235B和304SS相比,CSL在模拟混凝土孔溶液中钝化效率更高,但钝化膜中Fe2+/Fe3+比值较低导致其耐蚀性较差,CSL的临界氯离子浓度(0.16~0.2 mol/L)远小于Q235B(0.3 mol/L);氯离子的破钝效应主要影响试件表面的双电层结构,导致其有效电容增大,电荷转移电阻急剧下降;304SS的钝化膜为Fe和Cr的氧化物和羟基氧化物,具有更高的耐腐蚀性。Abstract: Aiming at the special P265GH low alloy steel (CSL) for nuclear power plant containment steel liner, the passivation and de-passivation effects of CSL in saturated Ca(OH)2 solution were investigated using electrochemical and X-ray photoelectron spectroscopy (XPS) analysis methods, and the corrosion resistance of CSL was compared with that of low carbon steel (Q235B) and 304 stainless steel (304SS). The results show that, compared with Q235B and 304SS, CSL has higher passivation efficiency in simulated concrete pore solution, but the low Fe2+/Fe3+ ratio in the passivation film leads to poor corrosion resistance, and the critical chloride ion concentration of CSL (0.16~0.2 mol/L) is much smaller than that of Q235b (0.3 mol/L). The de-passivation effect of chloride ions mainly affects the electric double layer structure on the surface of the specimen, which leads to the increase of its effective capacitance and the sharp decrease of charge transfer resistance. The passive film formed on the surface of 304SS is composed of Fe and Cr oxide and hydroxide, which has higher corrosion resistance.
-
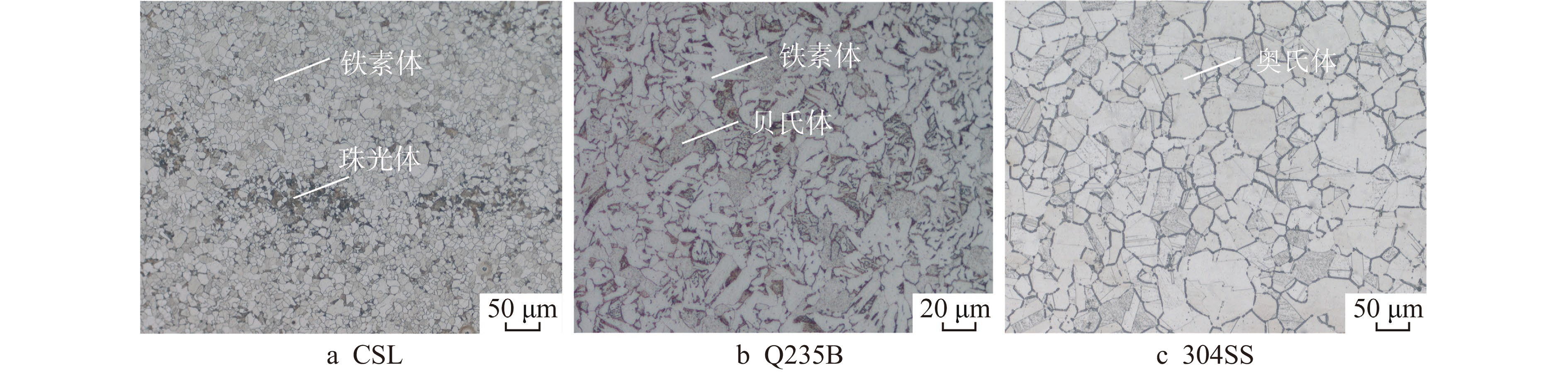
表 1 不同钢材的化学成分
Table 1. Chemical Composition of Different Steels
试件 化学成分/(质量百分数,%) Fe C Si Mn P S Cr Ni N Cu Alt Nb V Ti Mo CSL 余量 0.11 0.21 0.84 0.013 0.002 0.04 0.14 0.003 0.02 0.032 0.003 0.002 0.002 0.01 Q235B 余量 0.15 0.12 0.36 0.024 0.009 304SS 余量 0.034 0.45 1.42 0.018 0.003 18.1 8.13 0.054 表 2 氯离子浓度增长制度
Table 2. Schedule of the Increase of Chloride Concentration
阶段 时间/d 浓度梯度/
(mol·L−1)初始浓度/
(mol·L−1)终止浓度/
(mol·L−1)阶段一 6 0.01 0 0.06 阶段二 7 0.02 0.06 0.2 阶段三 6 0.05 0.2 0.5 阶段四 3 0.5 0.5 2.0 表 3 钝化阶段等效电路拟合参数值
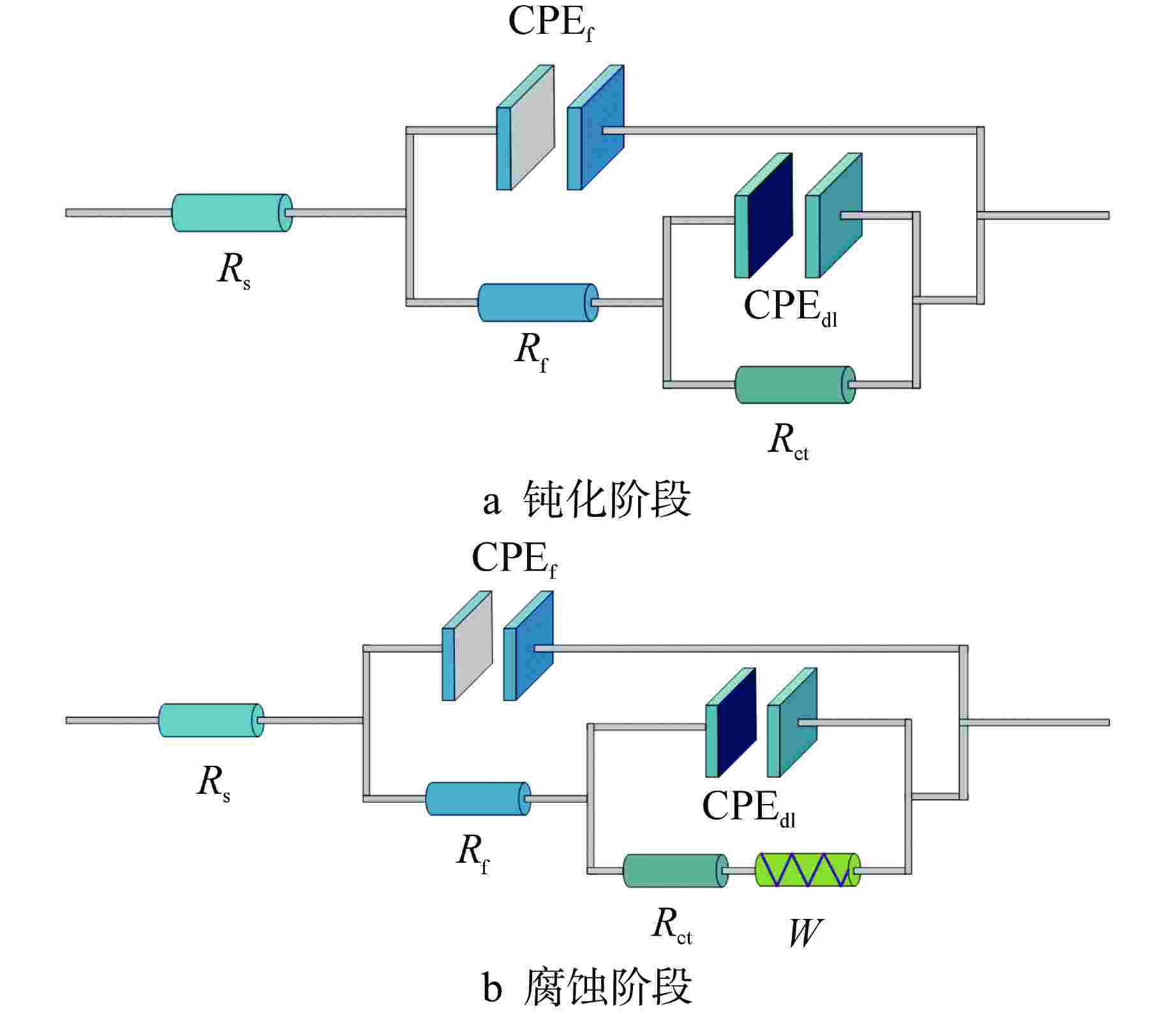
Table 3. EIS Fitting Values of Parameters of Equivalent Circuit in Passivation Stage
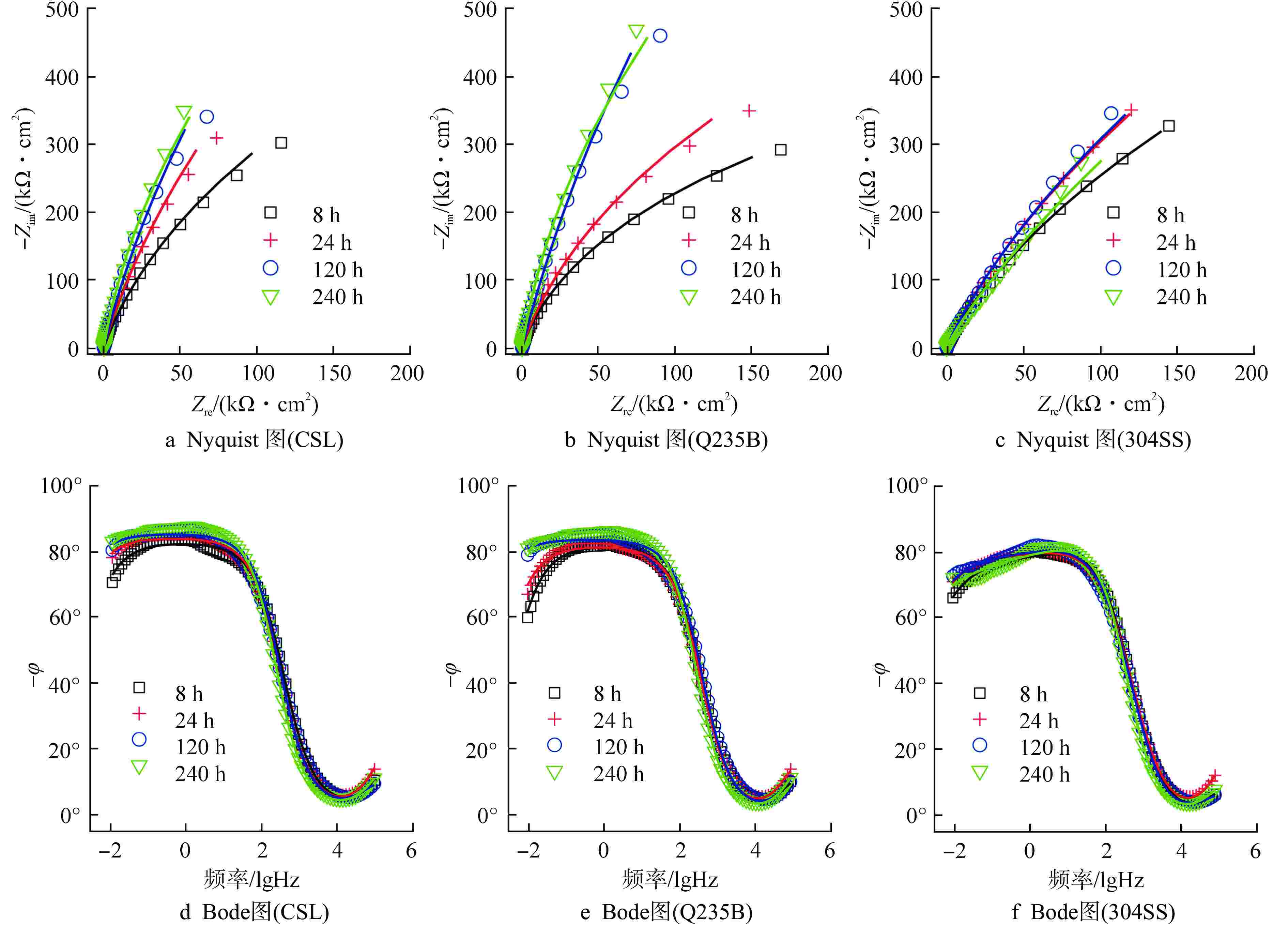
试件 钝化时间/h Rs/(Ω·cm2) CPEf Rf/(kΩ·cm2) CPEdl Rct/(kΩ·cm2) Qf /
(10−7 $ {\text{Ω}}^{{-1}}\cdot {\text{cm}}^{{-2}}\cdot {\text{s}}^{{n}_{\text{f}}}) $nf $C_{{\text{CPE}}_{\mathrm{f}}} $/
($ {\text{μF}} \cdot {\text{c}}{{\text{m}}^{ - 2}} $)Qdl/
(10−5 $ {{{\Omega }}^{{{ - 1}}}} \cdot {\text{c}}{{\text{m}}^{{{ - 2}}}} \cdot {{\text{s}}^{{n_{{\text{dl}}}}}} $)ndl $C_{{\text{CPE}}_{\mathrm{dl}}} $/
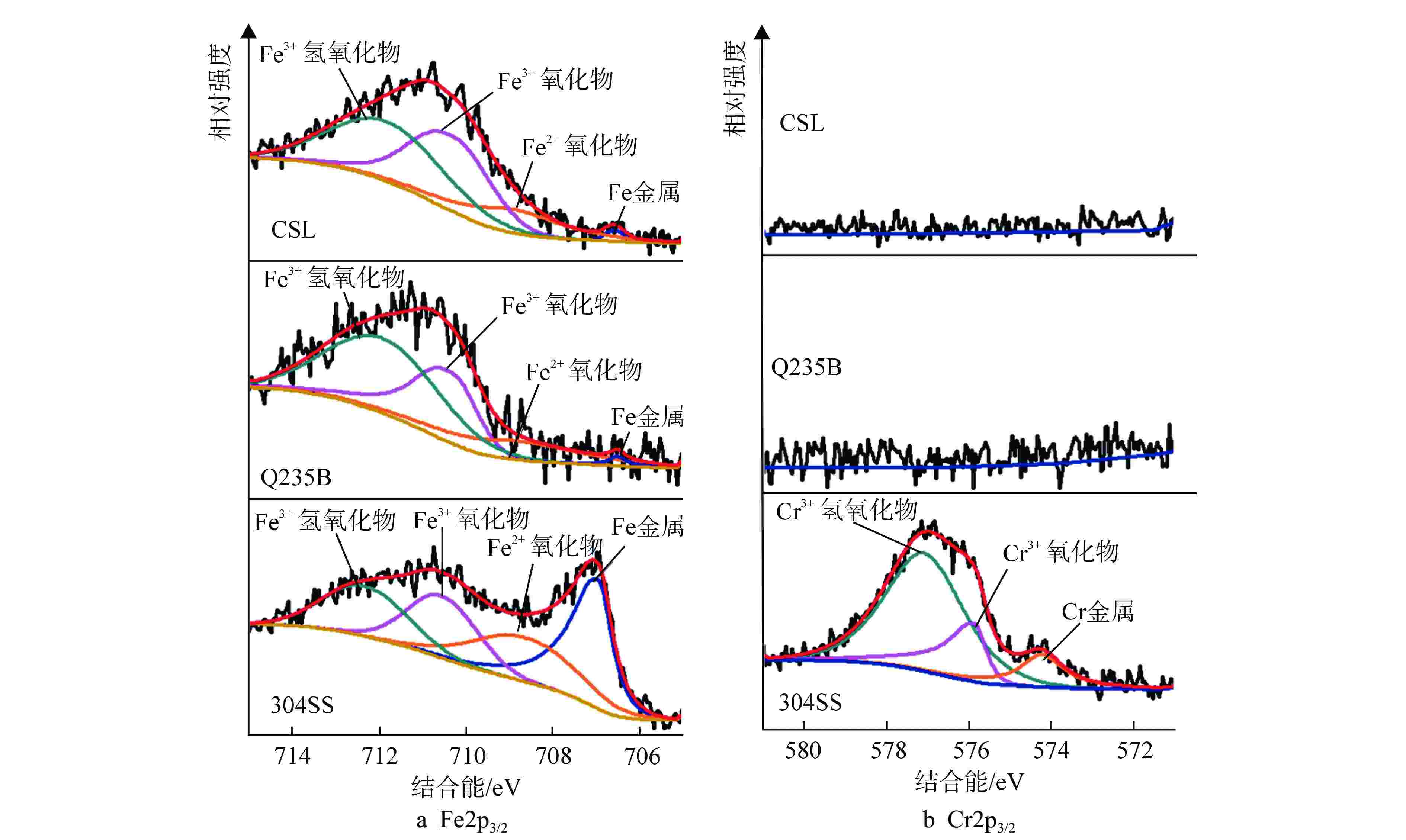
($ \mu{\text{F}} \cdot {\text{c}}{{\text{m}}^{ - 2}} $)CSL 8 14.95 1.48 1.00 1.48 11.37 8.60 0.90 30.81 1760.45 120 19.14 9.03 1.00 9.03 5.02 8.91 0.93 19.95 4469.18 240 16.93 2.68 1.00 2.68 9.09 9.00 0.95 16.32 3842.10 Q235B 8 17.35 4.08 1.00 4.08 7.84 7.71 0.91 22.89 926.93 120 18.21 5.41 1.00 5.41 7.37 6.64 0.92 16.98 5269.28 240 14.65 2.00 1.00 2.00 1.13 6.72 0.95 10.75 4252.95 304SS 8 26.50 9.29 1.00 9.29 52.70 10.86 0.79 9.32 2152.51 120 26.16 10.59 1.00 10.59 78.84 11.09 0.81 10.68 3623.63 240 26.16 13.93 1.00 13.93 52.70 10.03 0.75 8.08 4726.13 表中数据均为每组3个试件的平均值 表 4 Fe2+/Fe3+、FeOX/FeM 比值
Table 4. Values of Fe2+/Fe3+ and FeOX/FeM
试件 Fe2+/Fe3+ FeOX/FeM CSL 0.19 66.56 Q235B 0.21 49.01 304SS 0.61 1.89 表 5 腐蚀阶段等效电路拟合参数值
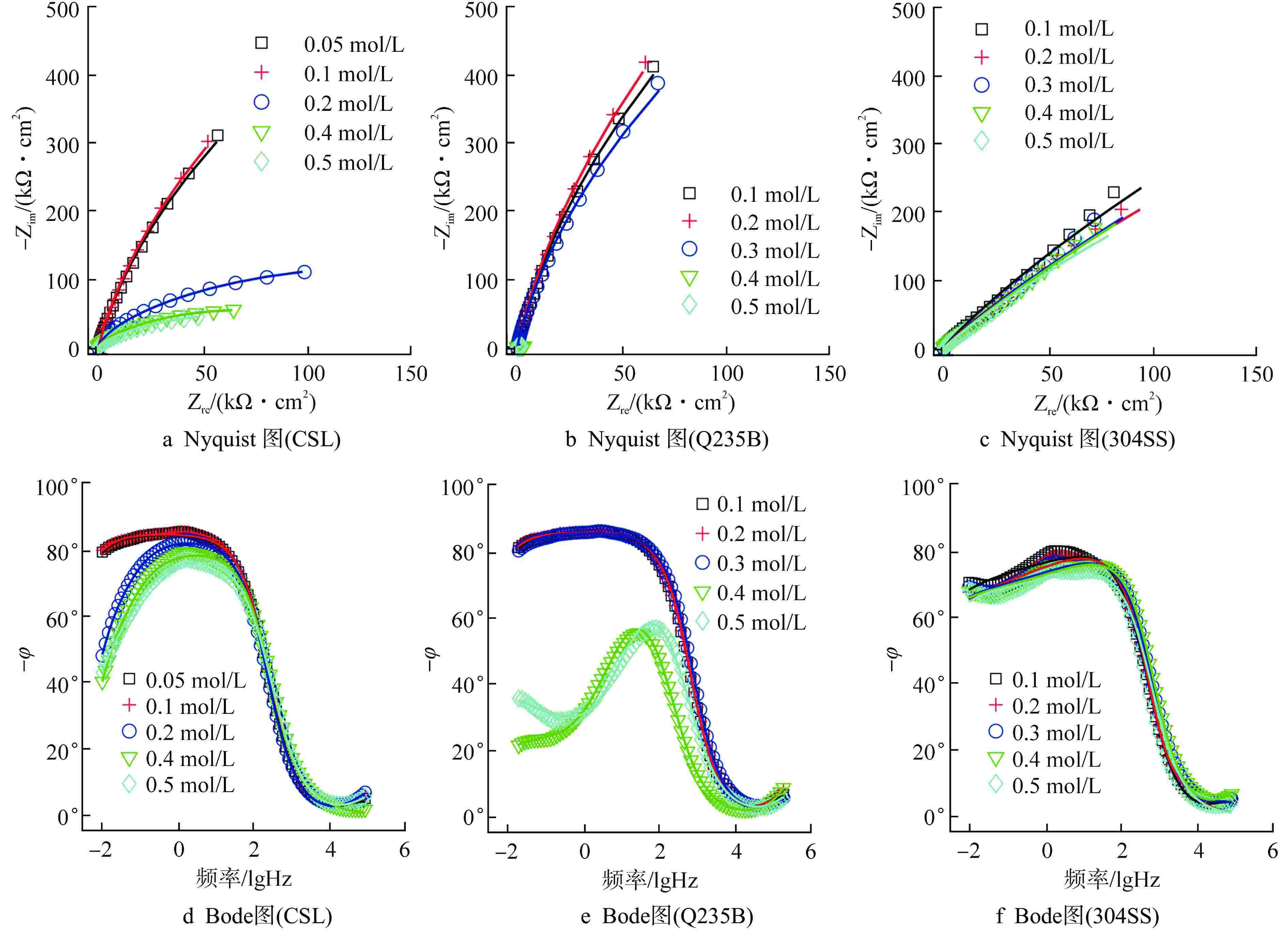
Table 5. EIS Fitting Values of Parameters of Equivalent Circuit in Corrosion Stage
试件 ${C_{{\text{C}}{{\text{l}}^{{ - }}}}}$/(mol·L−1) Rs/
(Ω·cm2)CPEf Rf /
(KΩ·cm2)CPEdl Rct/
(kΩ·cm2)Qf/
$ (10^{-7}\Omega^{-1}\cdot\text{c}\text{m}^{-2}\cdot\text{s}^{n_{\mathrm{f}}} $)nf $C_{{\text{CPE}}_{\mathrm{f}}} $/
$ (\text{μF}\cdot\text{c}\text{m}^{-2}) $Qdl /
$ \left(10^{-5}\Omega^{-1}\cdot\text{c}\text{m}^{-2}\cdot\text{s}^{n_{\mathrm{dl}}}\right) $ndl $C_{{\text{CPE}}_{\mathrm{dl}}} $/
$ (\text{μF}\cdot\text{c}\text{m}^{-2}) $CSL 0.1 18.71 18.2 1.00 18.2 3.15 10.00 0.94 19.37 3525.10 0.2 16.62 7.14 1.00 7.14 4.47 12.61 0.93 28.66 135.63 0.5 13.36 8.70 1.00 8.70 3.06 19.22 0.87 98.00 36.52 Q235B 0.3 14.81 12.00 1.00 12.00 3.09 7.92 0.95 13.48 3779.30 0.4 22.31 0.92 1.00 0.92 18.10 22.60 0.87 92.04 0.55 0.5 12.04 6.85 1.00 6.85 3.47 30.10 0.84 181.41 0.47 304SS 0.1 24.62 12.63 1.00 12.63 118.17 11.24 0.73 12.39 4848.30 0.4 28.18 9.47 1.00 9.47 5.19 15.09 0.70 5.07 5577.30 0.5 17.66 7.79 1.00 7.79 4.95 17.73 0.72 6.88 5681.30 表中数据均为每组3个试件的平均值;${C_{{\text{C}}{{\text{l}}^{{ - }}}}}$为氯离子浓度 -
[1] BERTOLINI L, ELSENER B, PEDEFERRI P, et al. Corrosion of steel in concrete: prevention, diagnosis, repair[M]. 2nd ed. Berlin: Wiley-VCH Verlag GmBH & Co. KGaA, 2013: 5-6.BERTOLINI L,ELSENER B,PEDEFERRI P,et al. Corrosion of steel in concrete: prevention,diagnosis,repair[M]. 2nd ed. Berlin: Wiley-VCH Verlag GmBH & Co. KGaA,2013:5-6. [2] GUO Q Q, WANG S X, CHEN S G, et al. Structural safety reliability of concrete buildings of HTR-PM in accidental double-ended break of hot gas ducts[J]. Nuclear Engineering and Technology, 2020, 52(5): 1051-1065. doi: 10.1016/j.net.2019.10.015 [3] ERLER B A, WEYERS R E, SAGUES A, et al. Nuclear containment steel liner corrosion workshop: final summary and recommendation report: SAND2010-8718; TRN: US1201117[R]. Albuquerque: Sandia National Laboratories, 2011. [4] DUNN D S, PULVIRENTI A L, HISER M A. Containment liner corrosion operating experience summary, technical letter report—revision 1:report of the United States Nuclear Regulatory Commission[R]. Rockville: U. S. Nuclear Regulatory Commission Office of Nuclear Regulatory Research, 2011. [5] PAEK Y, KIM S, YOON E, et al. Introduction of containment liner plate (CLP) corrosion[C]//Transactions of the Korean Nuclear Society Spring Meeting. Jeju:Korean Nuclear Society,2018. [6] CHOI W J, BAHN C B. Evaluation of corrosion behavior of carbon steel for containment liner plate according to environmental factors[C]//Transactions of the Korean Nuclear Society Spring Meeting. Jeju:Korean Nuclear Society,2019. [7] 廖开星,吴剑剑,李毅,等. 核电厂安全壳钢衬里的腐蚀防护与控制初探[J]. 腐蚀与防护,2019, 40(1): 61-65. doi: 10.11973/fsyfh-201901013 [8] 郭俊营,李忠诚,李文旭,等. 美国在役核电厂安全壳钢衬里锈蚀及修复技术研究进展[J]. 建筑结构,2022, 52(2): 127-134. [9] 曹光明,汤军舰,林飞,等. 典型氧化铁皮结构电化学腐蚀行为[J]. 中南大学学报: 自然科学版,2018, 49(6): 1366-1372. [10] 谢春洋,孔德军. 激光淬火对Cr12MoV钢渗硼层盐雾腐蚀和电化学腐蚀的影响[J]. 中南大学学报: 自然科学版,2016, 47(8): 2614-2620. [11] 陈杰,刘海霞,刘光磊,等. NaCl溶液腐蚀后304不锈钢的超声空蚀特征[J]. 中南大学学报: 自然科学版,2021, 52(5): 1436-1445. [12] 施锦杰,孙伟,耿国庆. 模拟混凝土孔溶液对钢筋钝化的影响[J]. 建筑材料学报,2011, 14(4): 452-458. doi: 10.3969/j.issn.1007-9629.2011.04.004 [13] ABD EL HALEEM S M, ABD EL AAL E E, ABD EL WANEES S, et al. Environmental factors affecting the corrosion behaviour of reinforcing steel: I. the early stage of passive film formation in Ca(OH)2 solutions[J]. Corrosion Science, 2010, 52(12): 3875-3882. doi: 10.1016/j.corsci.2010.07.035 [14] 苑旭雯. 模拟混凝土孔隙液中不锈钢自然钝化及脱钝行为研究[D]. 合肥: 中国科学技术大学,2021. [15] LIU M, CHENG X Q, LI X G, et al. Corrosion behavior of Cr modified HRB400 steel rebar in simulated concrete pore solution[J]. Construction and Building Materials, 2015, 93: 884-890. doi: 10.1016/j.conbuildmat.2015.05.073 [16] DE ROJAS R R. New developments in steel reinforcement protection from corrosion[M]. Massachusetts: Massachusetts Institute of Technology, 2001: 29-31.DE ROJAS R R. New developments in steel reinforcement protection from corrosion[M]. Massachusetts: Massachusetts Institute of Technology,2001:29-31. [17] SHI J J, MING J, WU M. Electrochemical behavior and corrosion products of Cr-modified reinforcing steels in saturated Ca(OH)2 solution with chlorides[J]. Cement and Concrete Composites, 2020, 110: 103587. doi: 10.1016/j.cemconcomp.2020.103587 [18] SHI J J, MING J, SUN W. Electrochemical behaviour of a novel alloy steel in alkali-activated slag mortars[J]. Cement and Concrete Composites, 2018, 92: 110-124. doi: 10.1016/j.cemconcomp.2018.06.004 [19] 明静,施锦杰,孙伟. 混凝土中低合金钢筋腐蚀产物的微结构分析[J]. 建筑材料学报,2020, 23(2): 347-353. [20] SERDAR M, MERAL C, KUNZ M, et al. Spatial distribution of crystalline corrosion products formed during corrosion of stainless steel in concrete[J]. Cement and Concrete Research, 2015, 71: 93-105. doi: 10.1016/j.cemconres.2015.02.004 [21] SUN J L, TANG H J, WANG C L, et al. Effects of alloying elements and microstructure on stainless steel corrosion: a review[J]. Steel Research International, 2022, 93(5): 2100450. doi: 10.1002/srin.202100450 [22] YUAN X W, WANG X, YANG H Y. Effects of pH and Cl− content on degradation process of pre-passivated stainless steels in an alkaline solution[J]. Journal of the Electrochemical Society, 2022, 169(3): 031506. doi: 10.1149/1945-7111/ac595b [23] CAO Y, GEHLEN C, ANGST U, et al. Critical chloride content in reinforced concrete—an updated review considering Chinese experience[J]. Cement and Concrete Research, 2019, 117: 58-68. doi: 10.1016/j.cemconres.2018.11.020 [24] The British Standards Institution. Flat products made of steels for pressure purposes-parts 2: non-alloy and alloy steels with specified elevated temperature properties: BS EN 10028-2: 2009[S]. 2017:6-9. [25] 李文旭. 核电厂安全壳钢衬里锈蚀后力学性能的试验研究[D]. 大连: 大连理工大学,2021. [26] 赵起越,范益,范恩点,等. 低合金结构钢腐蚀的影响因素及其耐蚀性判据[J]. 工程科学学报,2021, 43(2): 255-262. [27] 卢金马,黄俊铭,余波,等. 电极工作面积和孔隙液pH值对钢筋脱钝临界氯离子浓度的影响[J]. 建筑材料学报,2021, 24(5): 994-1001. doi: 10.3969/j.issn.1007-9629.2021.05.013 [28] SHI J J, WANG D Q, MING J, et al. Long-term electrochemical behavior of low-alloy steel in simulated concrete pore solution with chlorides[J]. Journal of Materials in Civil Engineering, 2018, 30(4): 04018042. doi: 10.1061/(ASCE)MT.1943-5533.0002194 [29] SHI J J, SUN W, JIANG J Y, et al. Influence of chloride concentration and pre-passivation on the pitting corrosion resistance of low-alloy reinforcing steel in simulated concrete pore solution[J]. Construction and Building Materials, 2016, 111: 805-813. doi: 10.1016/j.conbuildmat.2016.02.107 [30] LIU Y Q, SHI J J. Corrosion resistance of carbon steel in alkaline concrete pore solutions containing phytate and chloride ions[J]. Corrosion Science, 2022, 205: 110451. doi: 10.1016/j.corsci.2022.110451 [31] WANG D Q, MING J, SHI J J. Enhanced corrosion resistance of rebar in carbonated concrete pore solutions by Na2HPO4 and benzotriazole[J]. Corrosion Science, 2020, 174: 108830. doi: 10.1016/j.corsci.2020.108830 [32] YAO N, ZHOU X C, LIU Y Q, et al. Synergistic effect of red mud and fly ash on passivation and corrosion resistance of 304 stainless steel in alkaline concrete pore solutions[J]. Cement and Concrete Composites, 2022, 132: 104637. doi: 10.1016/j.cemconcomp.2022.104637 [33] LIU Y Q, ZHOU X C, GUAN X D, et al. Synergistic inhibition mechanism of phosphate and phytic acid on carbon steel in carbonated concrete pore solutions containing chlorides[J]. Corrosion Science, 2022, 208: 110637. doi: 10.1016/j.corsci.2022.110637 [34] LUO H, DONG C F, LI X G, et al. The electrochemical behaviour of 2205 duplex stainless steel in alkaline solutions with different pH in the presence of chloride[J]. Electrochimica Acta, 2012, 64: 211-220. doi: 10.1016/j.electacta.2012.01.025 [35] BURAK GUNAY H, GHODS P, BURKAN ISGOR O, et al. Characterization of atomic structure of oxide films on carbon steel in simulated concrete pore solutions using EELS[J]. Applied Surface Science, 2013, 274: 195-202. doi: 10.1016/j.apsusc.2013.03.014 [36] OMRAN M, FABRITIUS T, ELMAHDY A M, et al. XPS and FTIR spectroscopic study on microwave treated high phosphorus iron ore[J]. Applied Surface Science, 2015, 345: 127-140. doi: 10.1016/j.apsusc.2015.03.209 [37] GHODS P, BURKAN ISGOR O, BENSEBAA F, et al. Angle-resolved XPS study of carbon steel passivity and chloride-induced depassivation in simulated concrete pore solution[J]. Corrosion Science, 2012, 58: 159-167. doi: 10.1016/j.corsci.2012.01.019 [38] ANGST U, ELSENER B, LARSEN C K, et al. Critical chloride content in reinforced concrete—a review[J]. Cement and Concrete Research, 2009, 39(12): 1122-1138. doi: 10.1016/j.cemconres.2009.08.006 [39] ANDRADE C, ALONSO C. Corrosion rate monitoring in the laboratory and on-site[J]. Construction and Building Materials, 1996, 10(5): 315-328. doi: 10.1016/0950-0618(95)00044-5 [40] KOUŘIL M, NOVÁK P, BOJKO M. Threshold chloride concentration for stainless steels activation in concrete pore solutions[J]. Cement and Concrete Research, 2010, 40(3): 431-436. doi: 10.1016/j.cemconres.2009.11.005 -





 下载:
下载: