Study on Corrosion Resistance of ODS-FeCrAl Tube for ATF
-
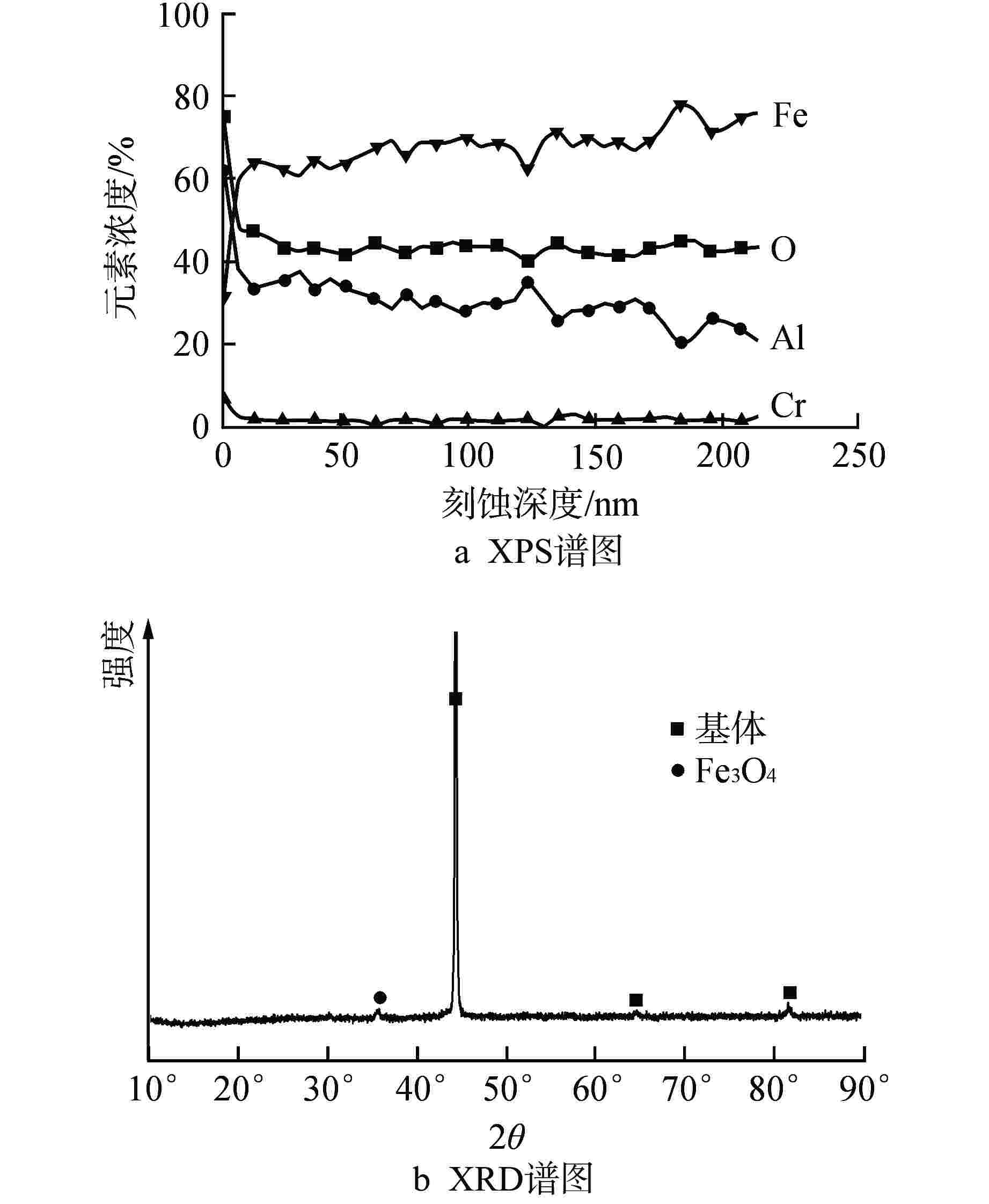
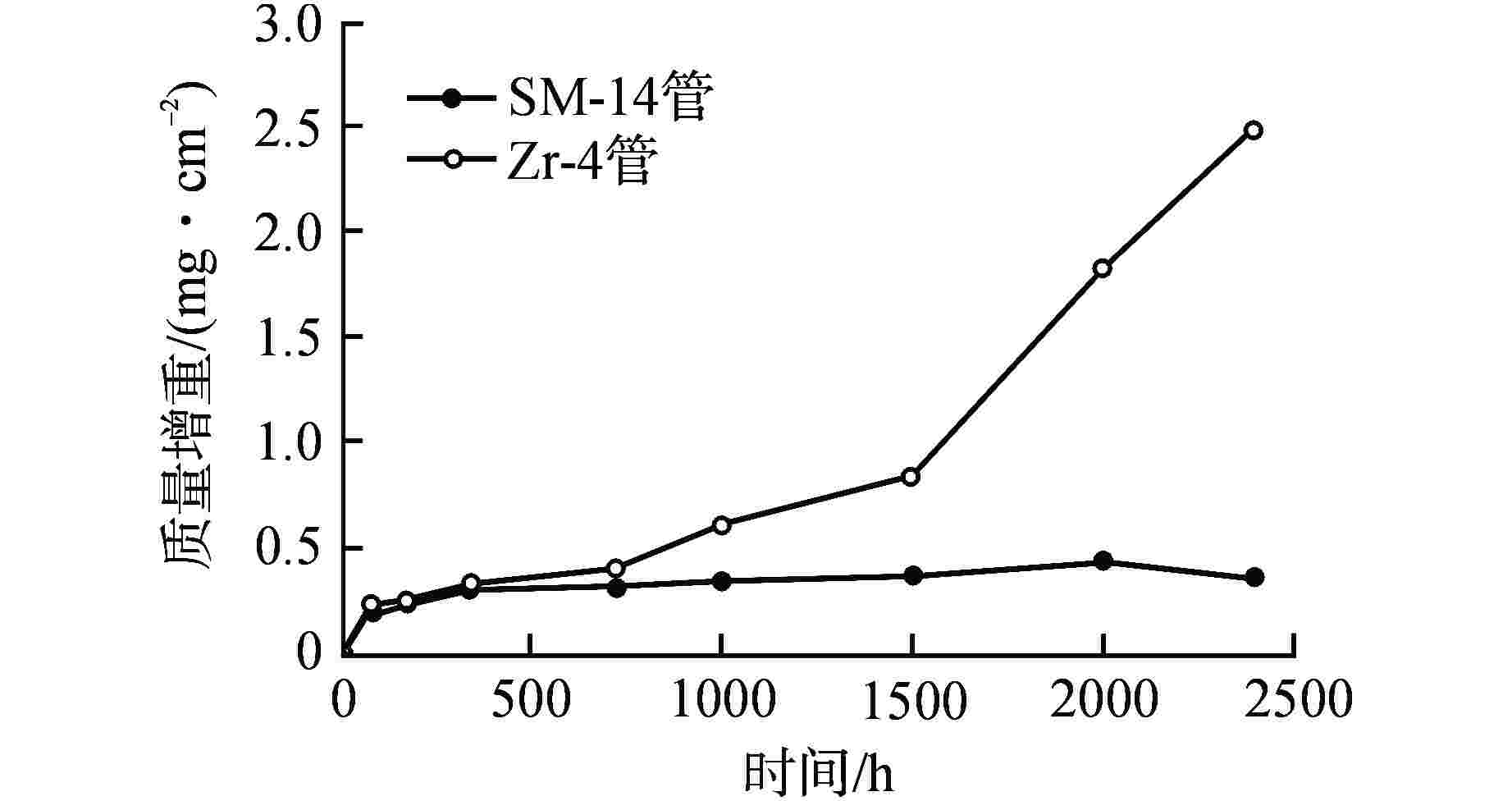
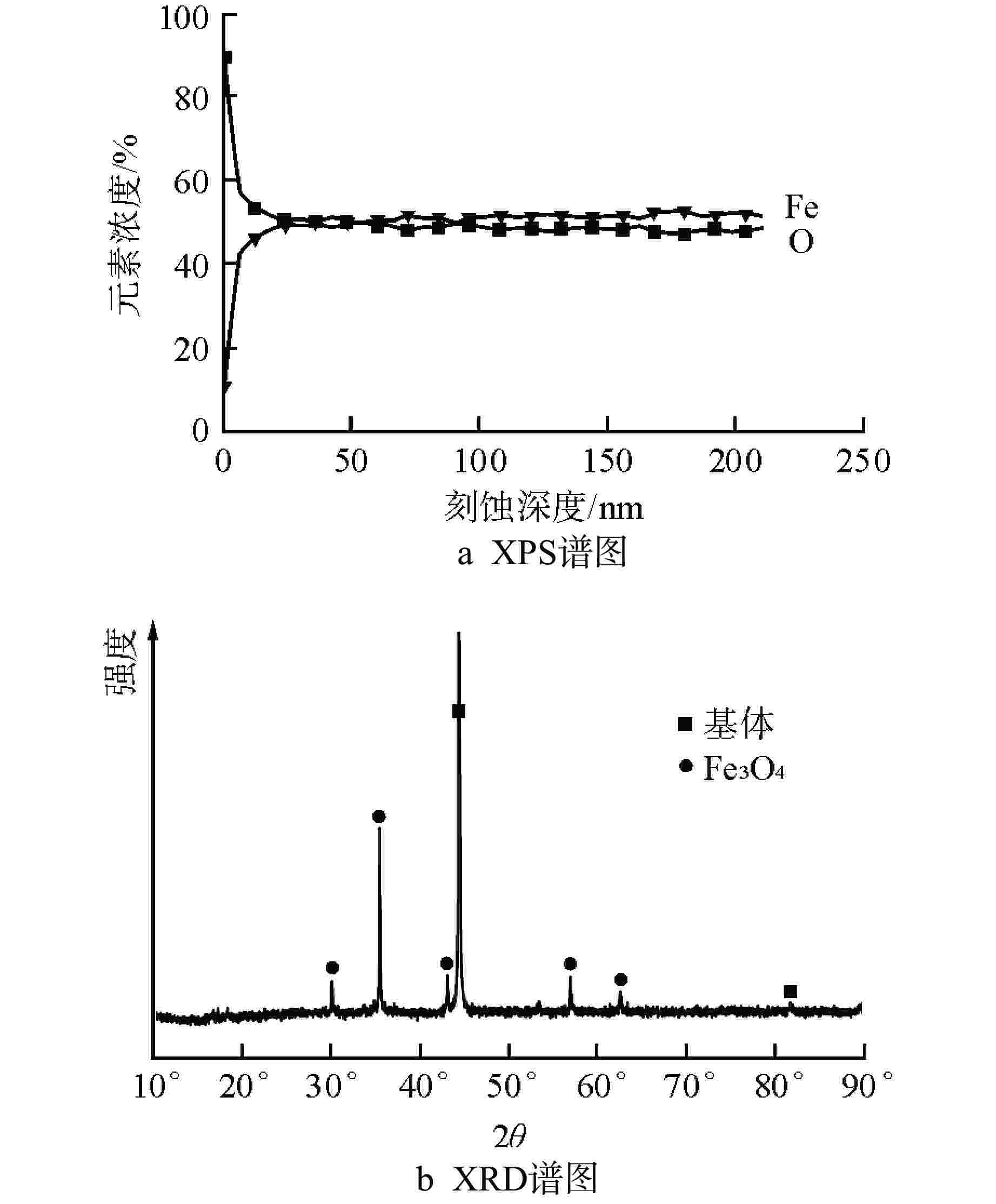
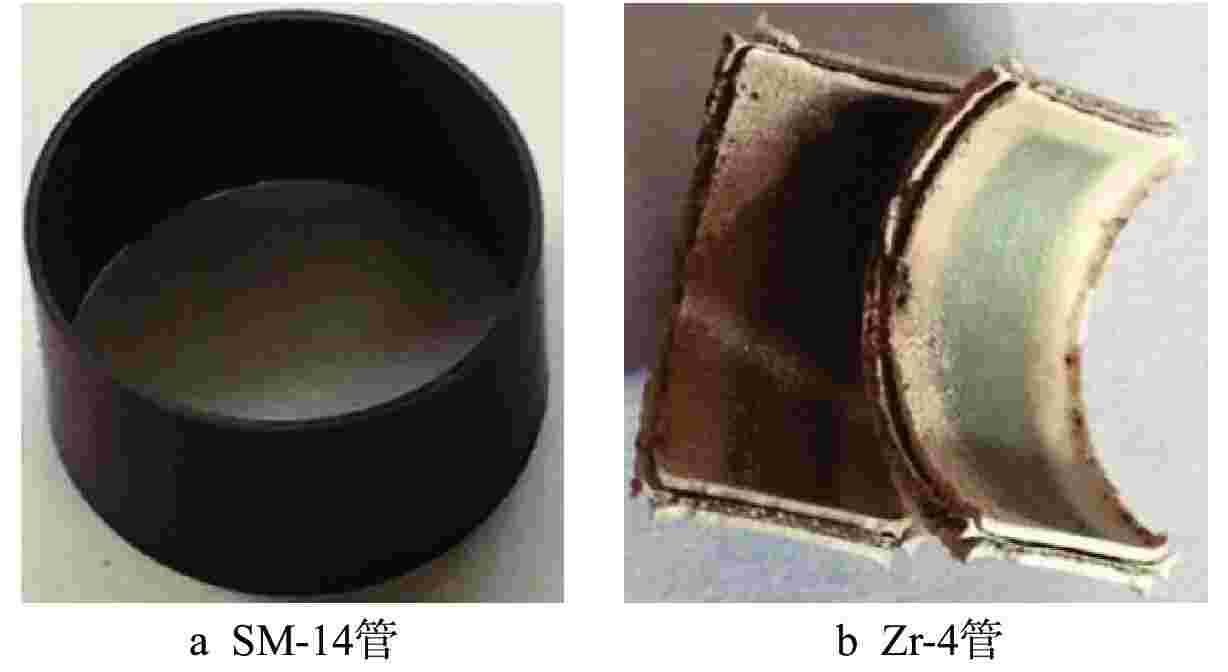
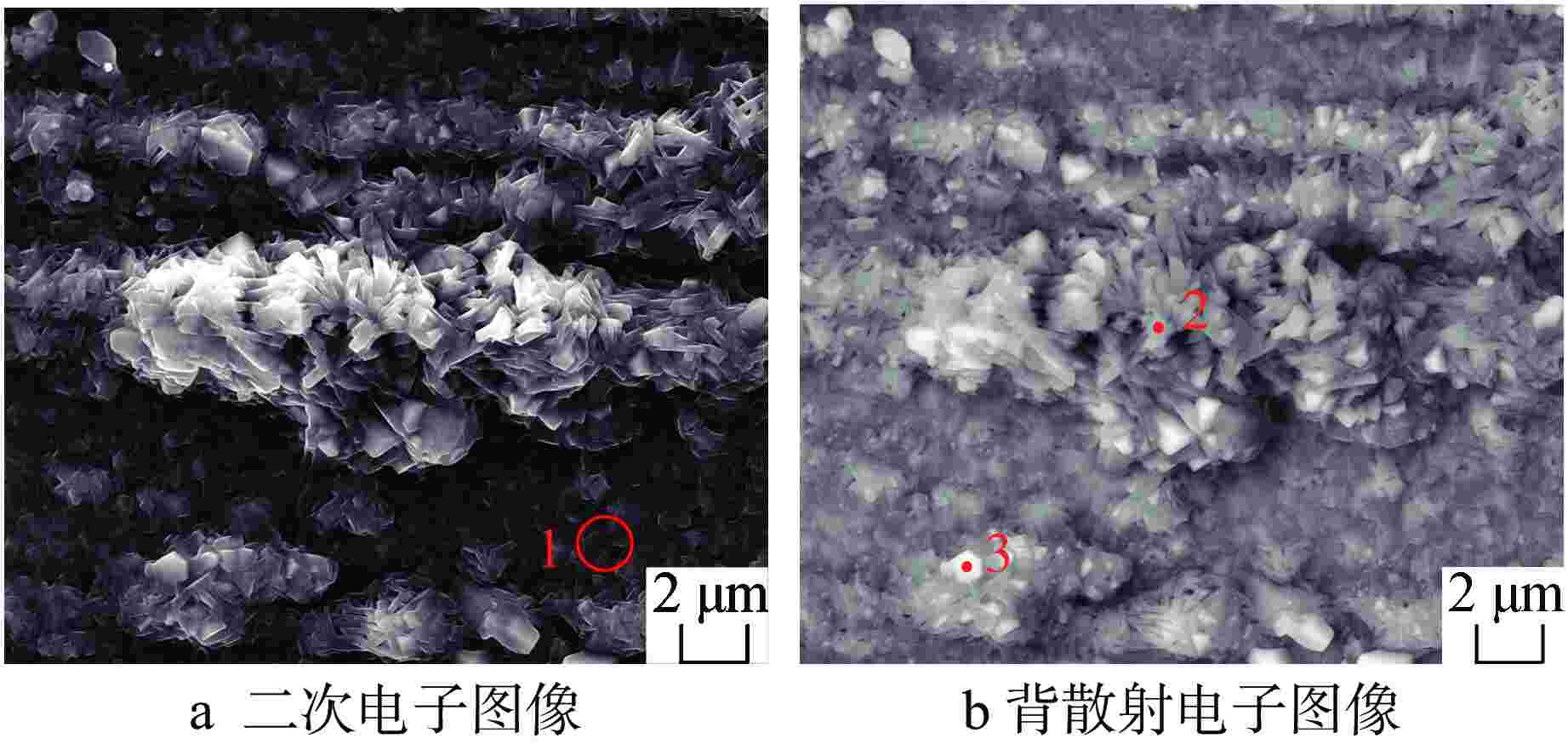
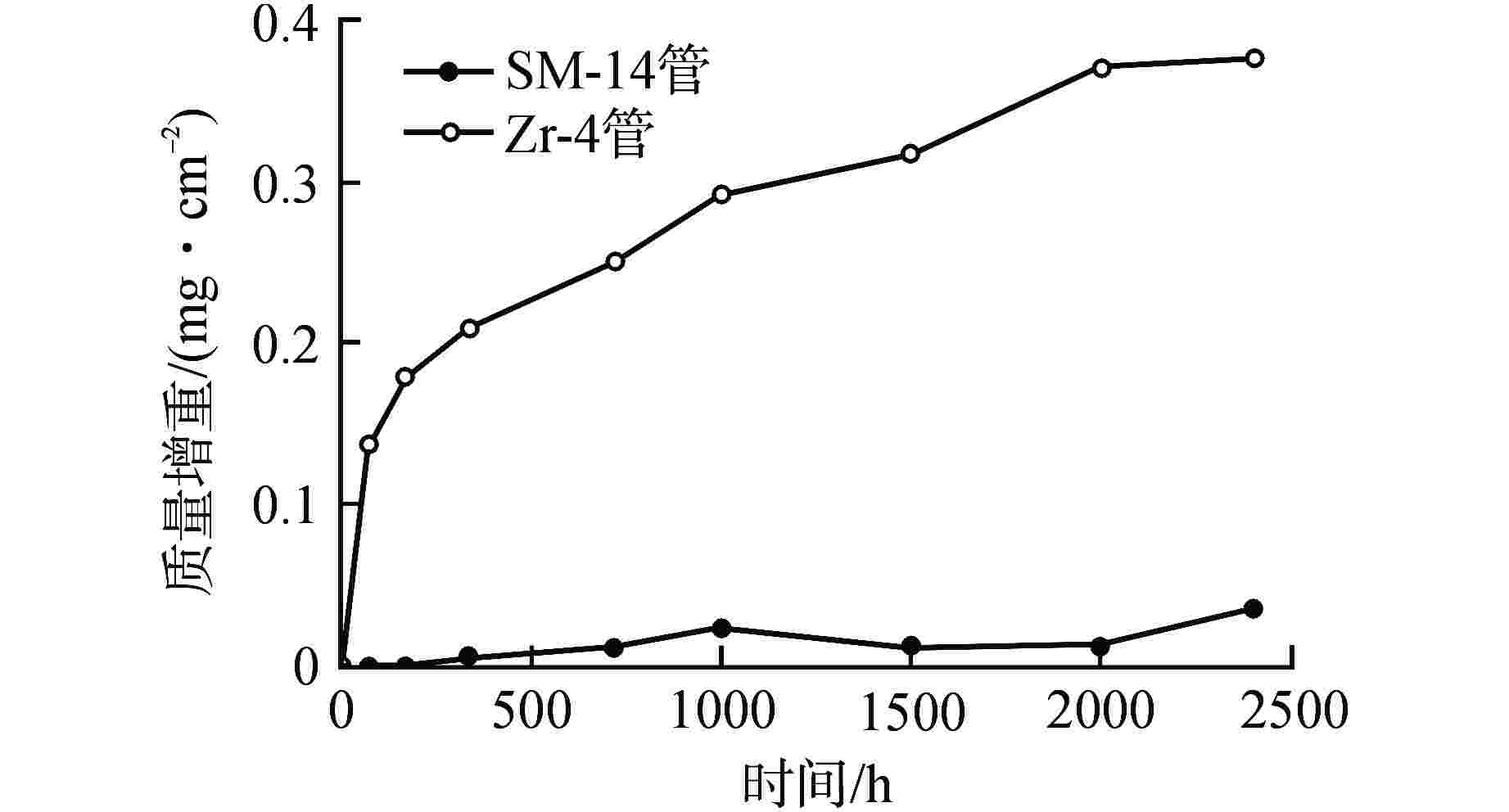
摘要: 针对尺寸为Φ9.5 mm×0.3 mm的氧化物弥散强化(ODS)-FeCrAl管材在360℃/18.6 MPa/100 d静态水溶液、360℃/18.6 MPa/1200 ppm B+2.2 ppm Li/100 d(1 ppm=10−6)动态水溶液、1200℃/0.1 MPa/8 h水蒸气中的腐蚀行为进行研究,利用扫描电镜(SEM)、X射线光电子能谱(XPS)和X射线衍射(XRD)等检测方法,分析管材表面氧化膜形貌、组织结构和元素分布。结果表明,360 ℃水溶液中极低的氧浓度使得ODS-FeCrAl管材在静态和动态水溶液的腐蚀产物主要是Fe3O4,质量增重分别为0.036 mg/cm2和0.36 mg/cm2,氧化膜厚度分别为管壁厚度的0.072%和0.72%;1200℃水蒸气腐蚀时,高温和充足的氧含量促使管材表面生成平均厚度为2.34 μm的α-Al2O3膜,延缓基体进一步氧化;腐蚀后的氧化膜表面和截面未发现开裂、孔洞等缺陷。与Zr-4管材参比试样相比,ODS-FeCrAl管材表现出优异的高温抗氧化、抗腐蚀性能。
-
关键词:
- ODS-FeCrAl管材 /
- 腐蚀 /
- 360℃水化学 /
- 1200℃水蒸气 /
- 氧化膜
Abstract: Oxide dispersion strengthened (ODS) FeCrAl alloy is one of the candidate materials for Accident Tolerant Fuel (ATF) cladding tubedue to its excellent mechanical strength, creep resistance and radiation-swelling resistance at elevated temperatures. The corrosion behaviors of ODS-FeCrAl tube with 9.5 mm outside diameter and 0.3 mm wall thickness in static water at 360 ℃ and18.6 MPa for 100 days, in flowing aqueous solution containing 1200 ppm B and 2.2 ppm Li at 360℃ and 18.6 MPa for 100 days and in steam at 1200℃ and 0.1 MPa for 8 hours, were studied herein. The morphology, composition and element distribution of oxide film were analyzed by SEM, XPS and XRD respectively. The corrosion products are mainly Fe3O4, due to the low oxygen content in both aqueous environments at 360℃. And the weight gain is 0.036 mg/cm2 and 0.36 mg/cm2, which corresponding oxide film thickness is 0.072% and 0.72% of that of tube wall respectively. In the steam at 1200℃, owing to high temperature and sufficient oxygen content, α-Al2O3 oxide film with a mean thickness of 2.34 μm is dominant on the surface, delaying further the oxidation of the matrix.No observable cracks and voids are identified on the surface and the cross section of oxide filmsin all corrosive environments.In comparison with Zr-4 reference cladding, ODS-FeCrAl tube exhibits an outstanding high temperature oxidation and corrosion resistance.-
Key words:
- ODS-FeCrAl tube /
- Corrosion /
- Water chemistry at 360 ℃ /
- Steam environment at 1200 ℃ /
- Oxidation film
-
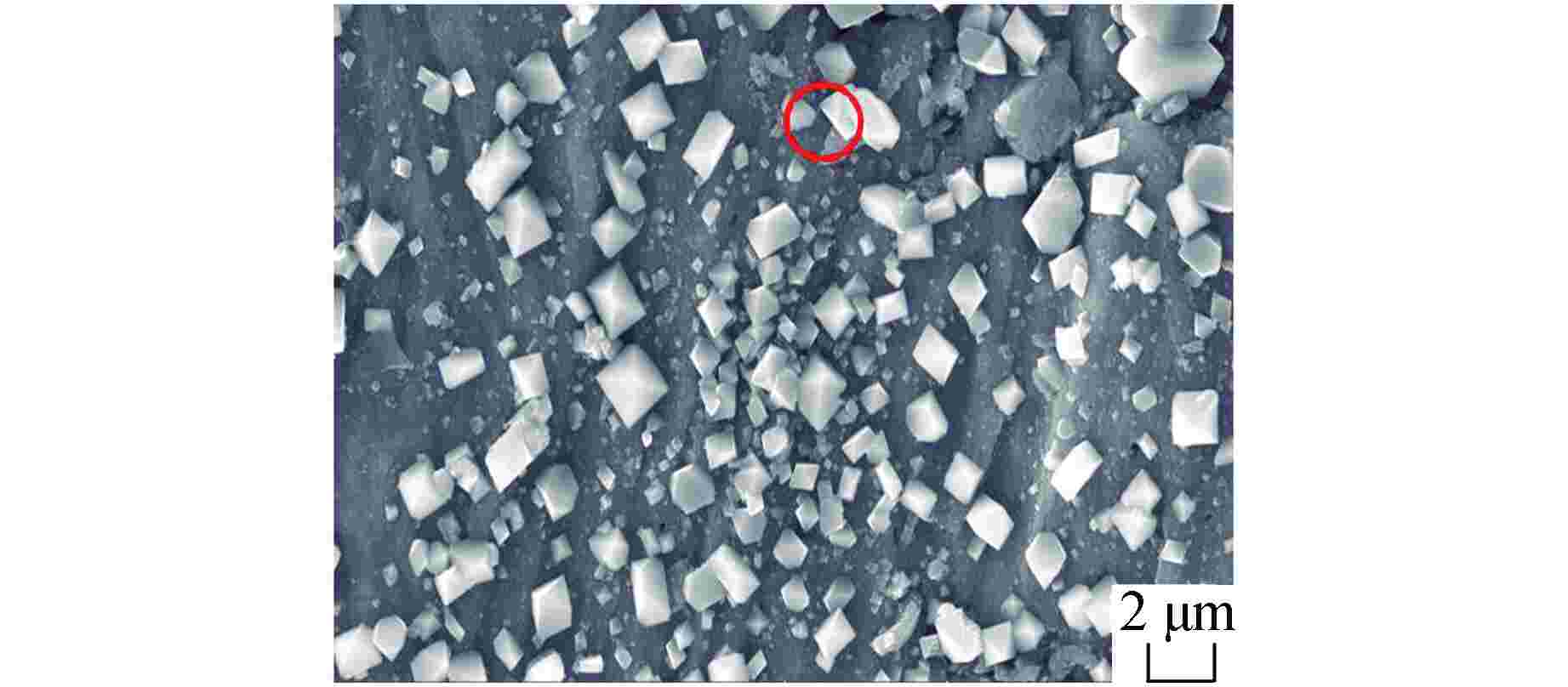
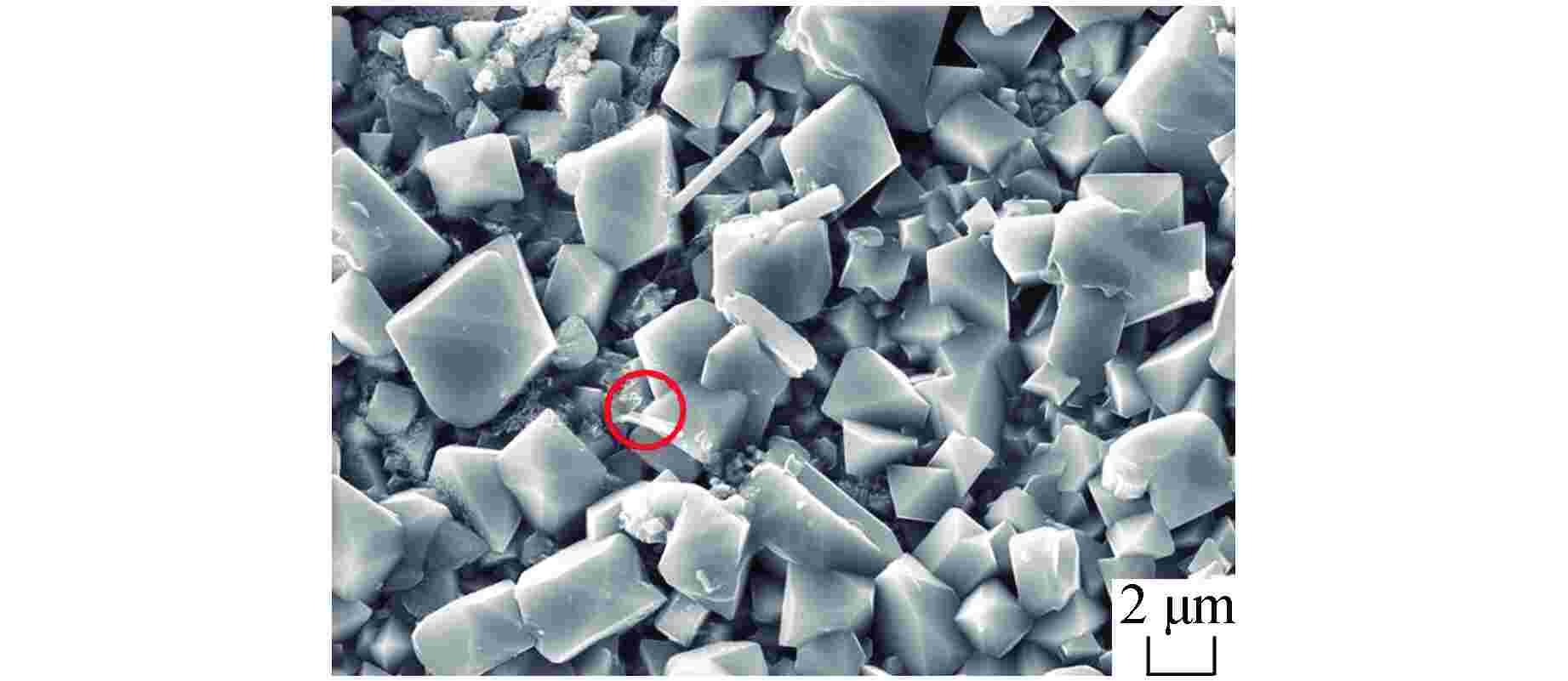
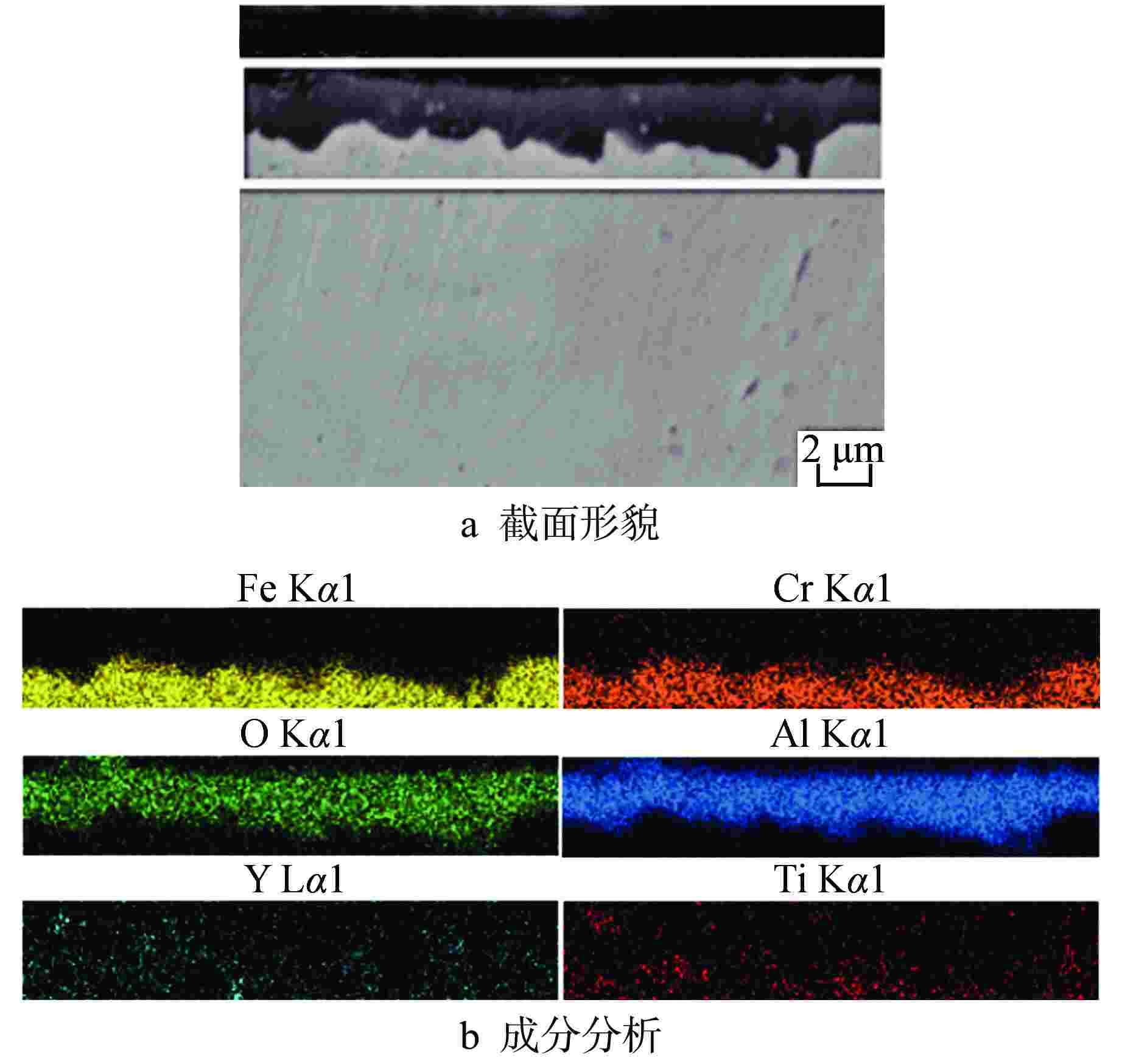
图 9 SM-14管经1200℃水蒸气腐蚀8 h后截面形貌及成分分析
图9a中白框为面扫区域
Figure 9. Morphology and Composition of Cross Section for SM-14 Tube after Steam Corrosion at 1200℃ for 8 Hours
表 1 360℃静态水腐蚀表面产物EDS分析结果
Table 1. EDS Analysis Results of Surface Products after Static Water Corrosion at 360℃
元素 质量百分比/% 原子百分比/% O 27.22 55.62 Al 2.27 2.75 Cr 8.27 5.20 Fe 62.24 36.43 表 2 360℃动态水腐蚀表面产物EDS分析结果
Table 2. EDS Analysis Results of Surface Products after Flowing Aqueous Solution Corrosion at 360℃
元素 质量百分比/% 原子百分比/% O 31.95 61.89 Al 0.45 0.52 Cr 1.81 1.08 Fe 65.79 36.51 表 3 1200℃水蒸气腐蚀8 h后表面产物EDS分析结果
Table 3. EDS Analysis Results of Surface Products after Steam Corrosion at 1200℃ for 8 h
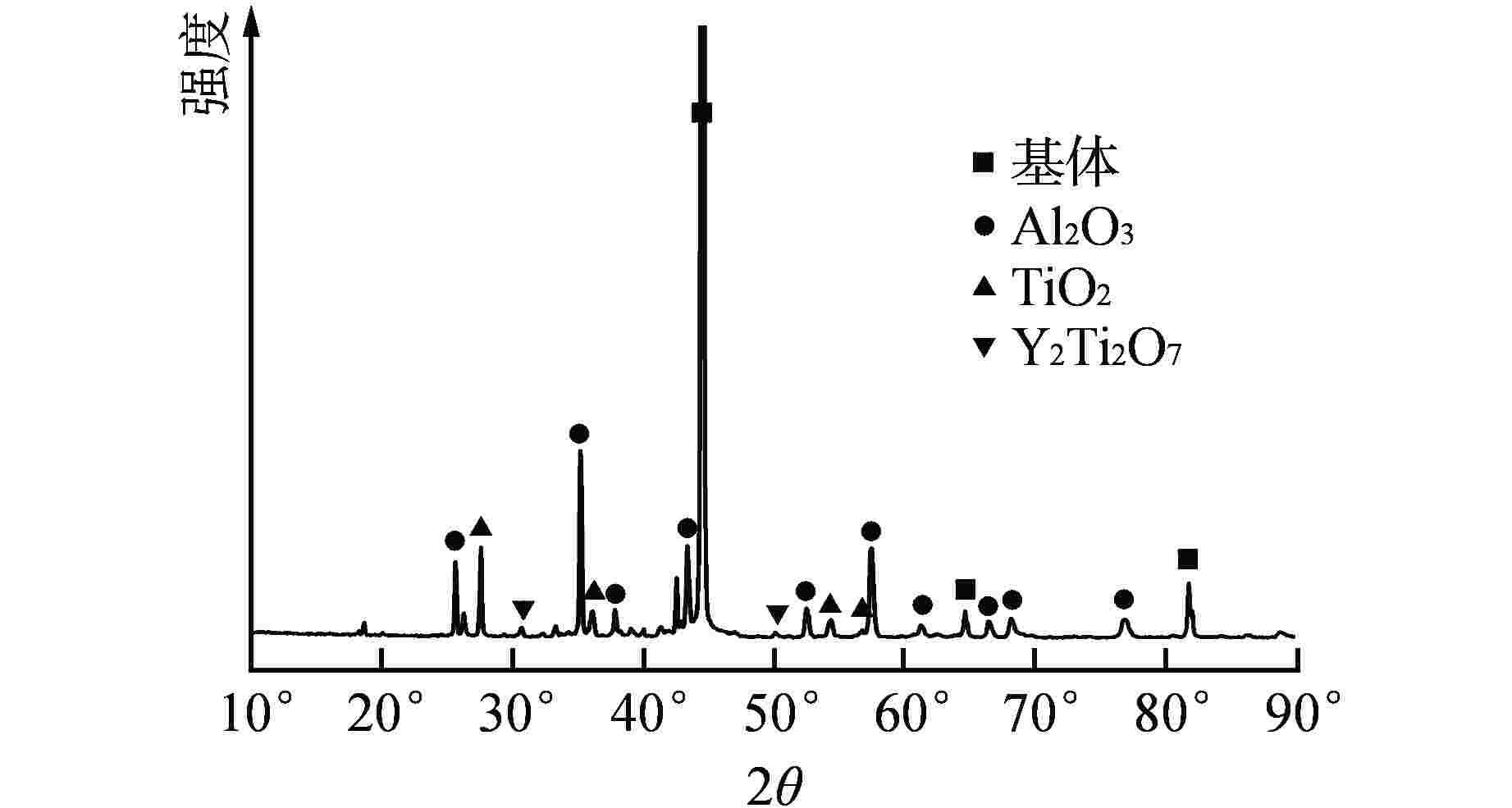
位置点 元素 质量百分比/% 原子百分比/% 1 O 38.48 54.96 Al 45.23 38.41 Ti 0.09 0.04 Cr 3.12 1.38 Fe 12.07 4.95 Y 1.01 0.26 2 O 48.88 63.69 Al 44.33 33.76 Ti 0.20 0.09 Cr 1.55 0.61 Fe 5.04 1.85 3 O 50.57 68.00 Al 29.43 23.46 Ti 12.39 5.57 Cr 1.43 0.59 Fe 6.18 2.38 -
[1] TERRANI K A. Accident tolerant fuel cladding development: Promise, status, and challenges[J]. Journal of Nuclear and Materials, 2018(501): 13-30. [2] DRYEPONDT S, UNOCIC K A, HOELZER D T. et al Development of low-Cr ODS FeCrAl alloys for accident-tolerant fuel cladding[J]. Journal of Nuclear Materials, 2018(501): 59-71. [3] PINT B A, UNOCIC K A. Steam oxidation evaluation of Fe-Cr alloys for accident tolerant nuclear fuel cladding[J]. Oxidation of Metals, 2017, 87(1-2): 515-526. [4] WU S, LI J, LI W, et al. Characterization of oxide dispersoids and mechanical properties of 14Cr-ODS-FeCrAl alloys[J]. Journal of Alloys and Compounds, 2020(814): 152282. [5] 李静, 吴飒建, 葛鹏, 等. 一种高温水蒸气腐蚀试验装置: 中国, ZL 201920633267.2[P]. 2020-03-31. [6] LIU T, WANG C, SHEN H, et al. The effects of Cr and Al concentrations on the oxidation behavior of oxide dispersion strengthened ferritic alloys[J]. Corrosion Science, 2013(76): 310-316. [7] BRIANT C L, LUTHRA K L. Surface segregation in MCrAIY alloys[J]. Metallurgical Transactions A, 1988, 19(8): 2099-2108. doi: 10.1007/BF02645212 [8] CHEN R Y, YEUN W Y D. Review of the high-temperature oxidation of Iron and carbon steels in air or oxygen[J]. Oxidation of Metals, 2003, 59(5/6): 433-468. doi: 10.1023/A:1023685905159 [9] KUANG W, WU X, HAN E H. Influence of dissolved oxygen concentration on the oxide film formed on 304 stainless steel in high temperature water[J]. Corrosion Science, 2012(63): 259-266. [10] 周邦新,李强,姚美意,等. 锆-4合金在高压釜中腐蚀时氧化膜显微组织的演化[J]. 核动力工程,2005, 26(4): 364-371. doi: 10.3969/j.issn.0258-0926.2005.04.014 [11] VANKEERBERGHEN M, WEYNS G, GAVRILOV S, et al. Crack propagation rate modelling for 316SS exposed to PWR-relevant conditions[J]. Journal of Nuclear Materials, 2009(384): 274-285. [12] TERRANI K A, PINT B A, KIM Y J, et al. Uniform corrosion of FeCrAl alloys in LWR coolant environments[J]. Journal of Nuclear Materials, 2016(479): 36-47. [13] HORITA T, YAMAJI K, YOKOKAWA H, et al. Effects of Si and Al concentrations in Fe–Cr alloy on the formation of oxide scales in H2-H2O[J]. International Journal of Hydrogen Energy, 2008(33): 6308-6315. [14] RAM D L, TATLOCK G J, FALKE U. Segregation of reactive elements at oxide grainboundaries in FeCrAlRE alloys[J]. Materials at High Temperatures, 2005, 22(3-4): 497-503. doi: 10.1179/mht.2005.060 [15] MOLINS R, GERMIDIS A, ANDRIEU E. Oxidation of thin FeCrAlstrips: kinetic and microstructural studies[J]. Microscopy of Oxidation-3, 1996(16-18): 3-11. [16] DRYEPONDT S, TURAN J, LEONARD D, et al. Long-term oxidation testing and lifetime modelingof cast and ODS FeCrAl alloys[J]. Oxidation of Metals, 2017, 87(1-2): 215-248. doi: 10.1007/s11085-016-9668-2 -





 下载:
下载: