Research on Deoxygenation Behavior of Sulfite Deoxygenation Resin
-
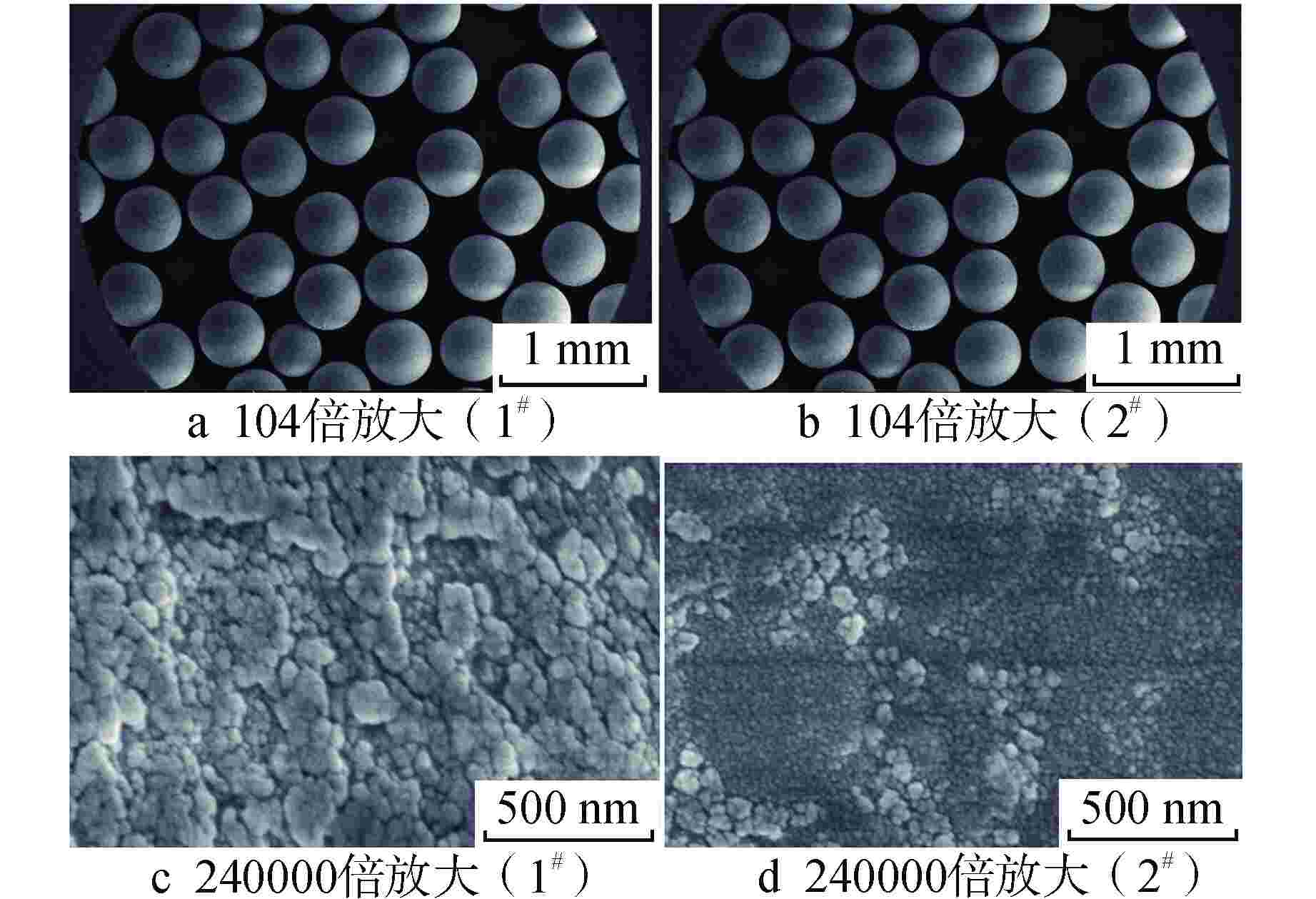
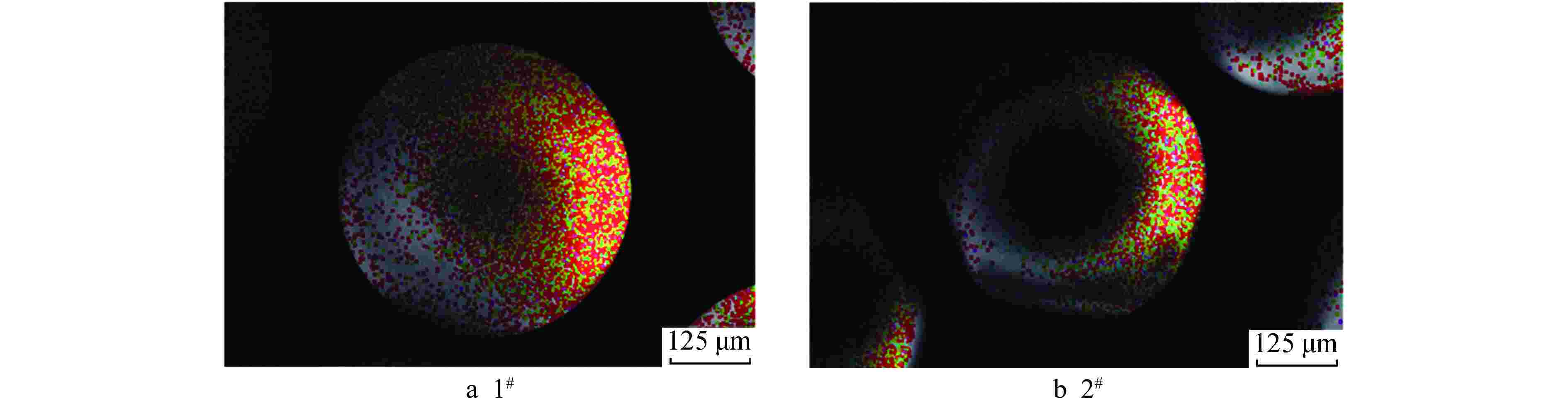
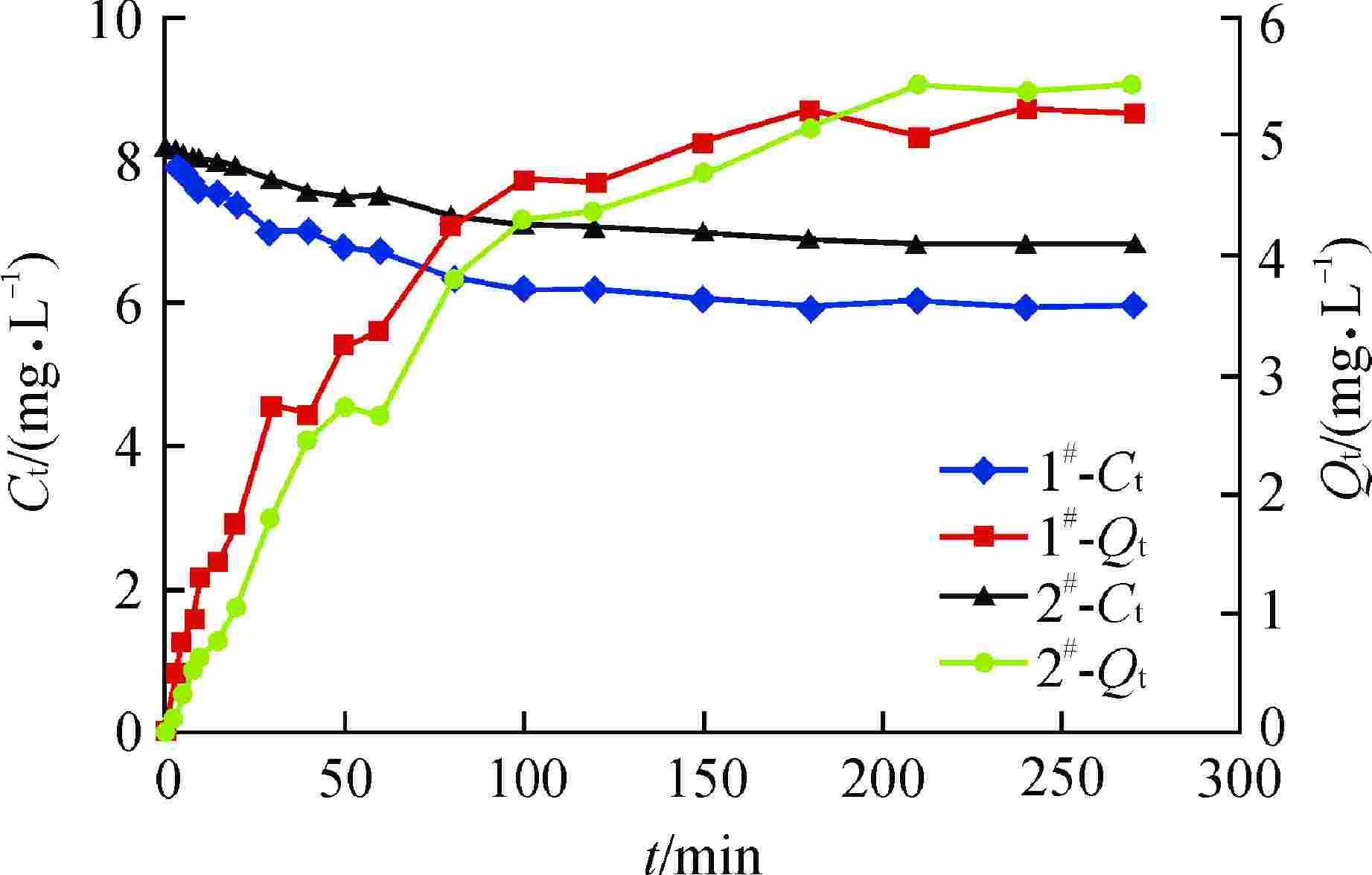
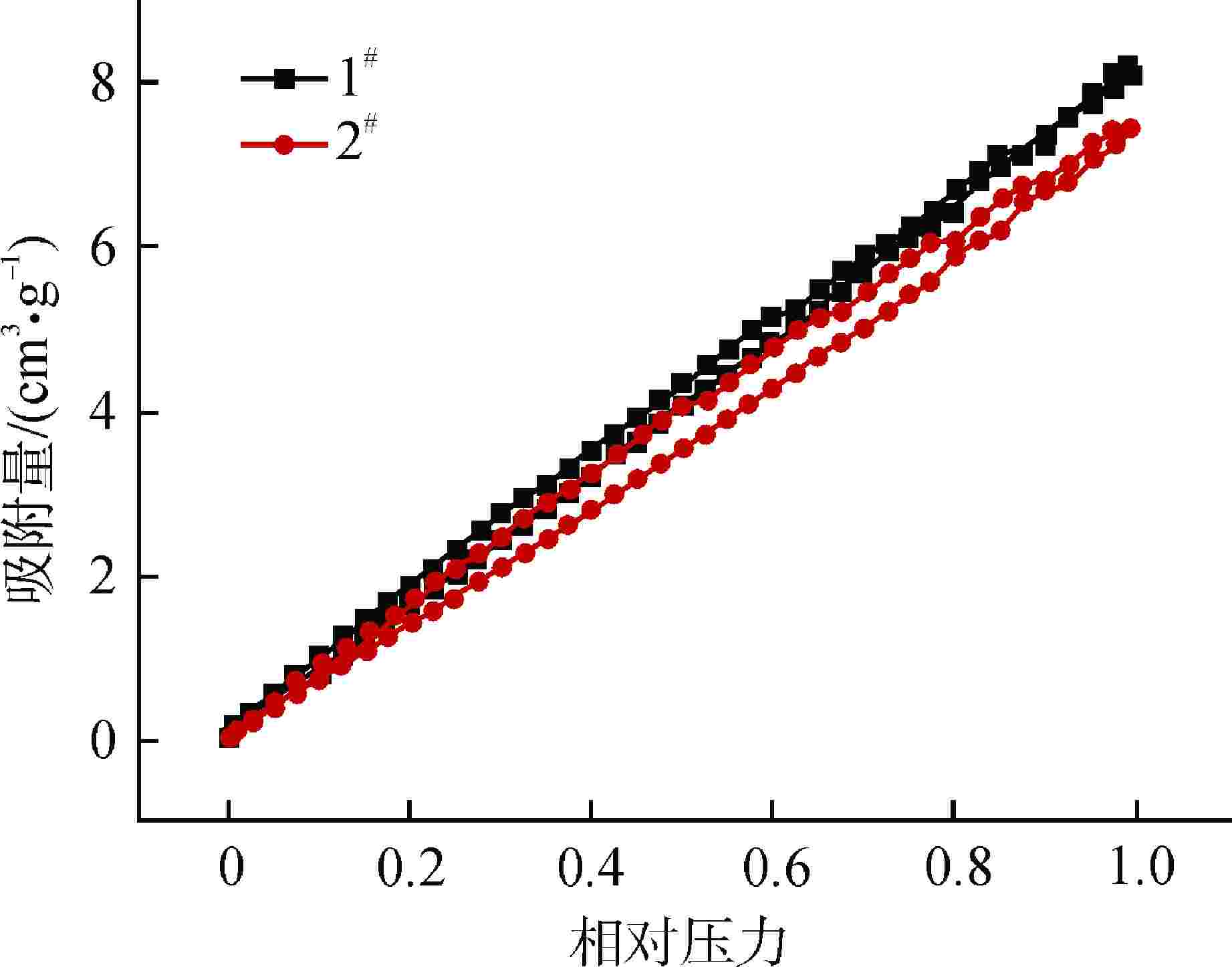
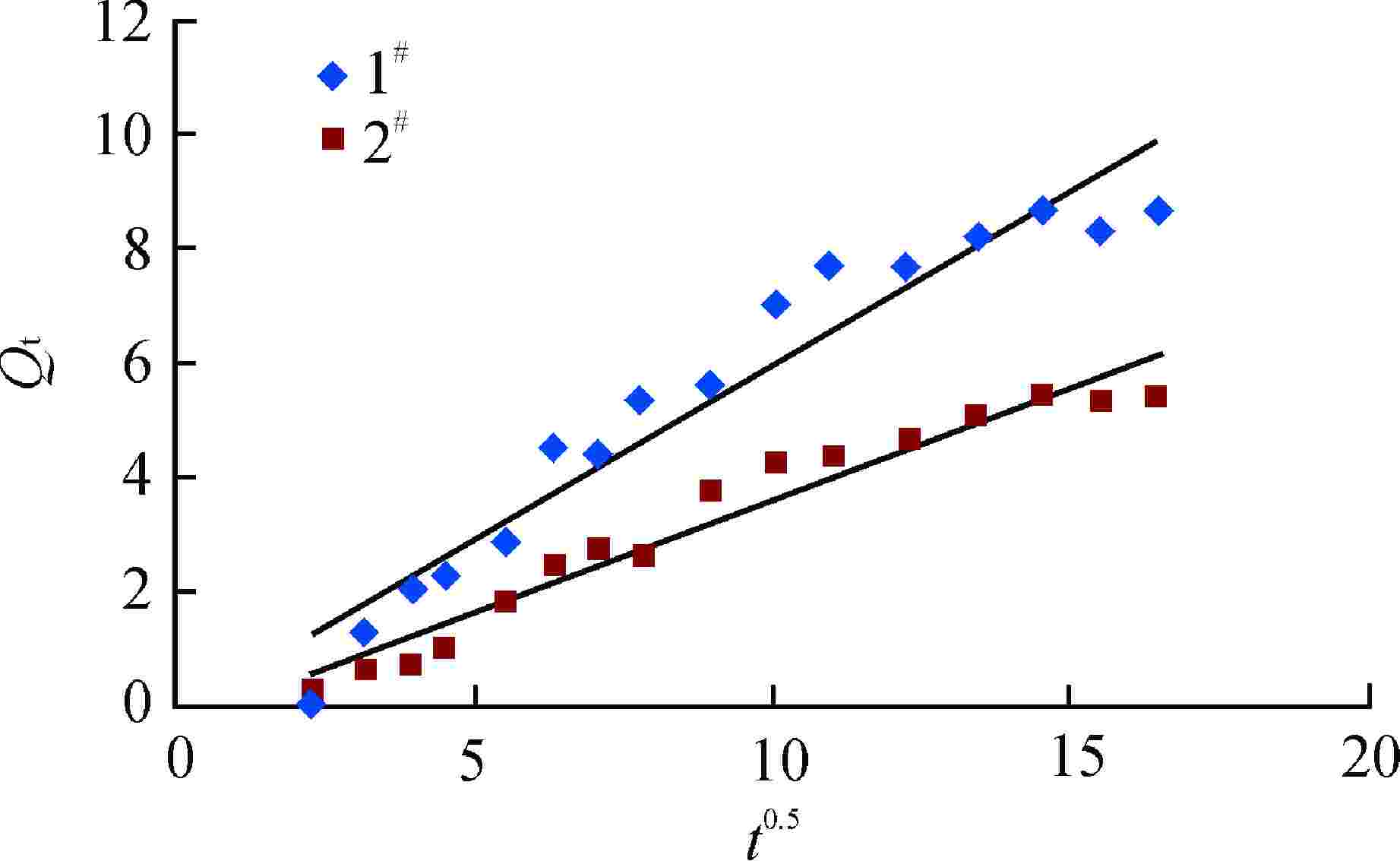
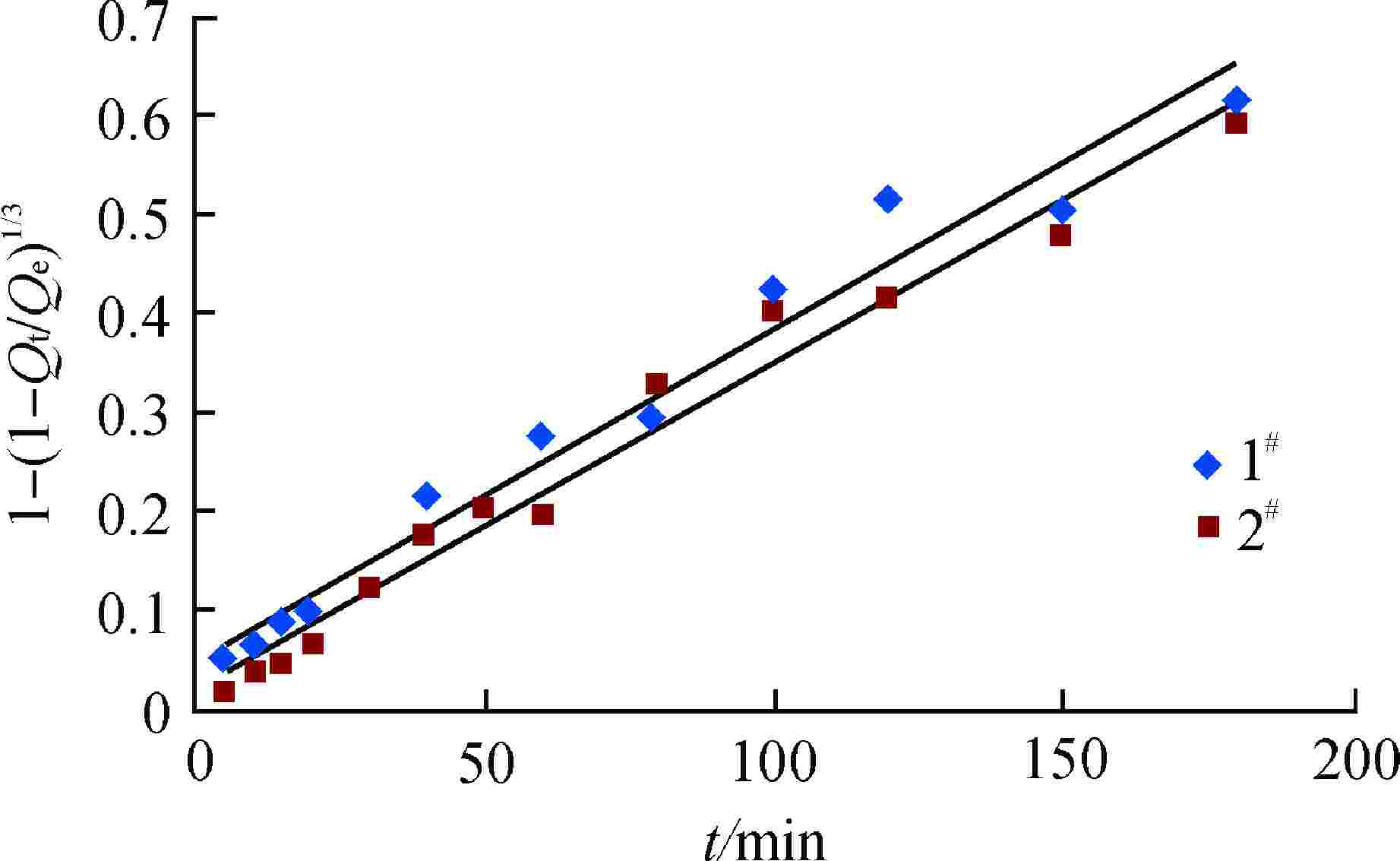
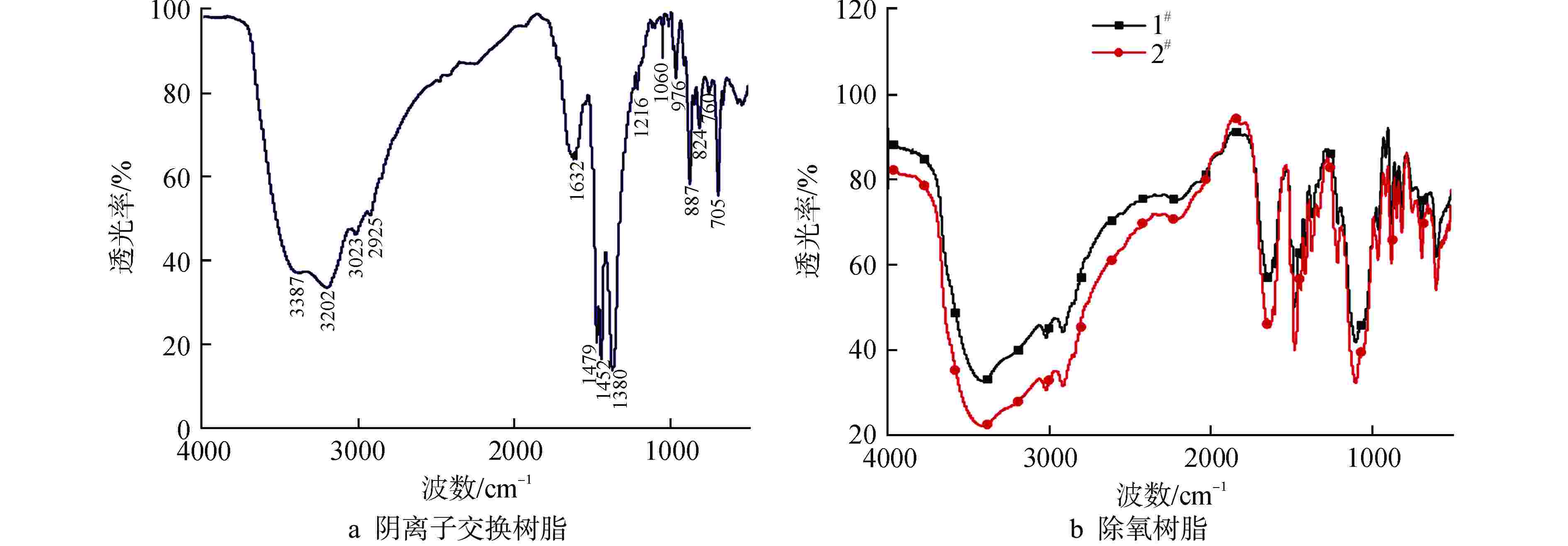
摘要: 亚硫酸型除氧树脂的除氧性能与其结构、吸附动力学热力学关系密切,除氧机理的研究可以为树脂的后续优化提供理论指导。采用红外光谱法、扫描电子显微镜和色散谱分析表征除氧树脂的微观结构,开展了亚硫酸型除氧树脂的除氧动力学、除氧热力学研究。微观表征判断除氧树脂是以碳链为骨架的聚苯乙烯亚硫酸型树脂。除氧树脂对水中溶解氧的吸附动力学过程符合伪二级动力学模型,水中溶解氧的吸附过程存在化学吸附。扩散传质模型拟合显示耐高温除氧树脂的主要控制步骤为化学反应,阴离子树脂转型的亚硫酸型除氧树脂的主要控制步骤为液膜扩散。除氧树脂的吉布斯自由能变(ΔG)<0、焓变(ΔH)>0、熵变(ΔS)>0,表明其吸附为可逆的自发进行的吸热反应,升高温度有利于吸附的进行。本文研究结果可以为除氧树脂的性能优化提供指导。Abstract: The deoxygenation performance of sulfite deoxygenation resin is closely related to its structure and adsorption kinetics thermodynamics. The study of deoxygenation mechanism can provide theoretical guidance for the subsequent optimization of resin. The microstructure of deoxygenation resin was characterized by infrared spectroscopy, scanning electron microscope and dispersion spectrum analysis, and the deoxygenation kinetics and thermodynamics of sulfite deoxygenation resin were studied. The microscopic characterization shows that the deoxygenation resin is a polystyrene sulfurous acid resin with carbon chain as the skeleton. The adsorption kinetic process of deoxygenation resin on the dissolved oxygen in aqeous solution conforms to the pseudo-second-order kinetic model, and there is chemical adsorption in the adsorption process of dissolved oxgen in water. The diffusion mass transfer model fitting showed that the main control step of the high-temperature deoxygenation resin was chemical reaction, and the main control step of sulfite deoxygenation resin transformed from anionic resin was liquid film diffusion. Gibbs free energy change (ΔG)<0, enthalpy change (ΔH)>0 and entropy change (ΔS)>0 of the deoxygenation resin indicate that its adsorption is a reversible spontaneous endothermic reaction, and increasing the temperature is beneficial to the adsorption. The research results in this paper can provide guidance for the performance optimization of deoxygenation resin.
-
Key words:
- Sulfite deoxygenation resin /
- Dissolved oxygen /
- Adsorption equilibrium /
- Kinetics /
- Thermodynamics
-
表 1 1#除氧热力学参数
Table 1. Results of Deaeration Thermodynamic Parameters of 1#
T/K ΔG/(kJ·mol−1) ΔH/(kJ·mol−1) ΔS/[J·(mol·K)−1] 298 −21.365 33.12 196.26 303 −26.347 308 −27.328 313 −28.309 T—温度;ΔG—吉布斯自由能变;ΔH—焓变;ΔS—熵变 表 2 2#除氧热力学参数
Table 2. Results of Deaeration Thermodynamic Parameters of 2#
T/K ΔG/(kJ·mol−1) ΔH/(kJ·mol−1) ΔS/[J·(mol·K)−1] 298 –0.802 68.28 231.82 303 –1.961 308 -–3.120 313 –4.279 -
[1] 范赏,吴旭东,姜磊,等. 除盐水加氢催化除氧试验研究[J]. 湿法冶金,2022,41(1):97-91. doi: 10.13355/j.cnki.sfyj.2022.01.016 [2] 陈海松. 浅析催化加氢除氧技术在AP1000核电厂中的应用[J]. 给水排水,2017,53(S2):23-25. [3] 张昊,刘芬芬,孙海军,等. 亚硫酸型除氧树脂的热分解产物研究[J]. 舰船科学技术,2011,33(8):41-44. [4] MOGHIMI F, JAFARI A H, YOOZBASHIZADEH H, et al. Adsorption behavior of Sb(III) in single and binary Sb(III)−Fe(II) systems on cationic ion exchange resin: adsorption equilibrium, kinetic and thermodynamic aspects[J]. Transactions of Nonferrous Metals Society of China, 2020, 30(1): 236-248. doi: 10.1016/S1003-6326(19)65195-2 -





 下载:
下载: