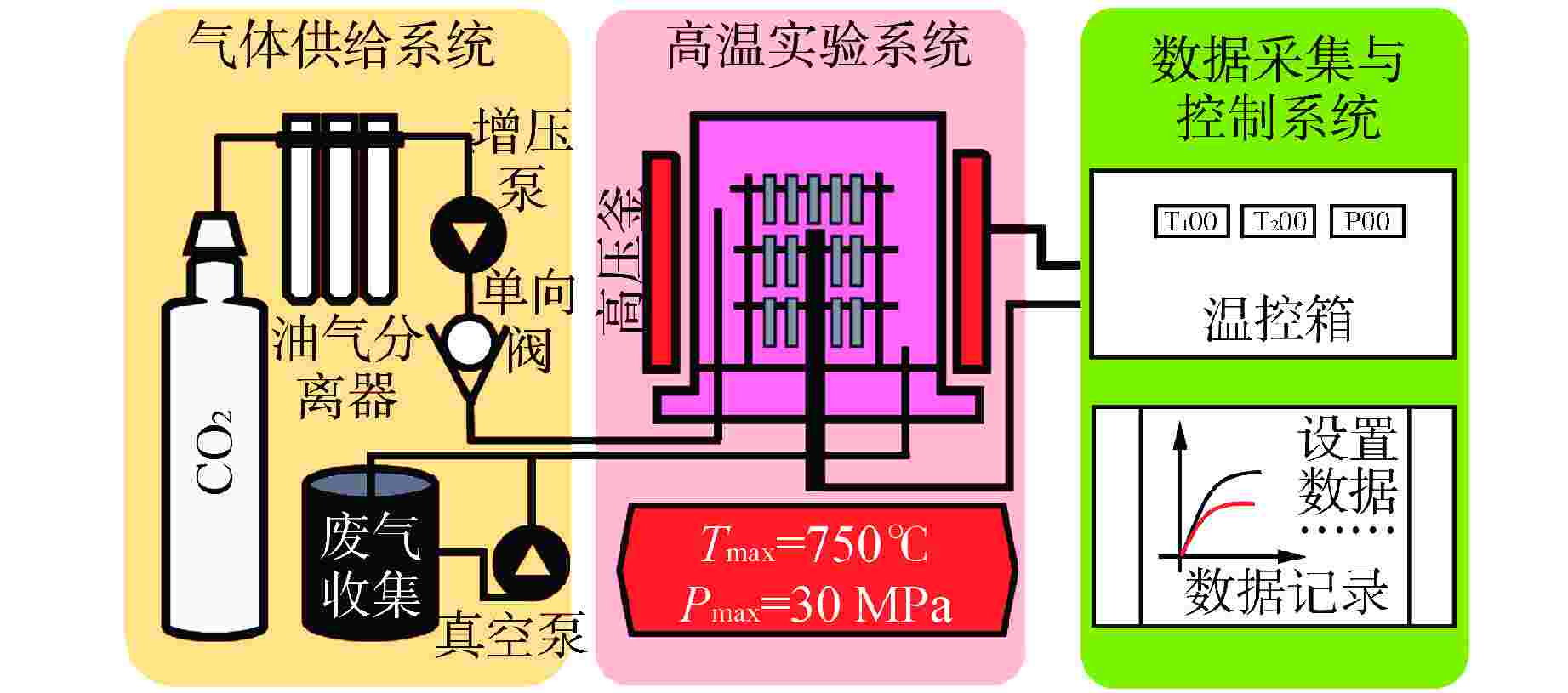
Study on Uniform Corrosion Behavior of 600 Alloy and 304 Stainless Steel in Supercritical Carbon Dioxide Environment
-
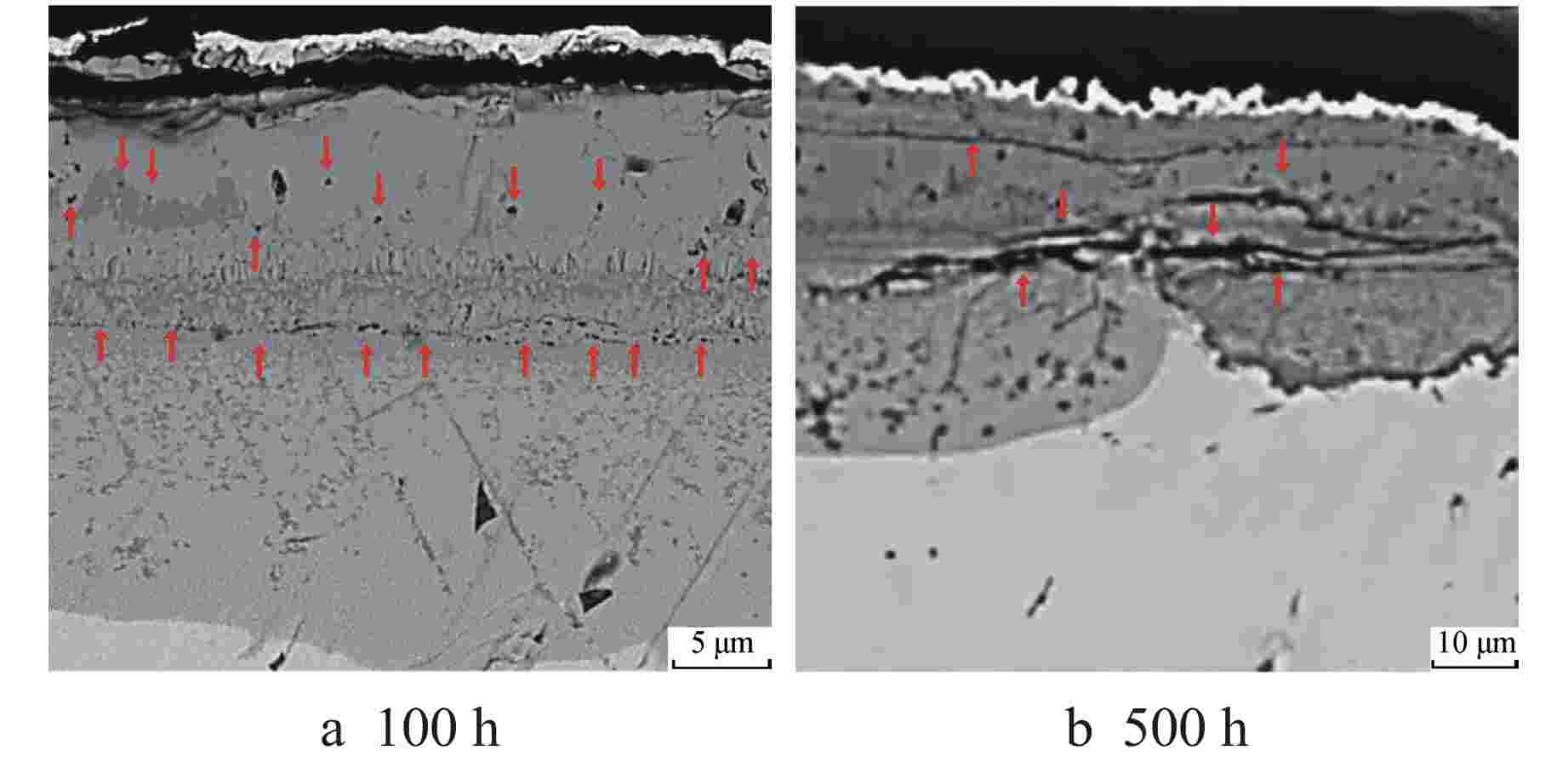
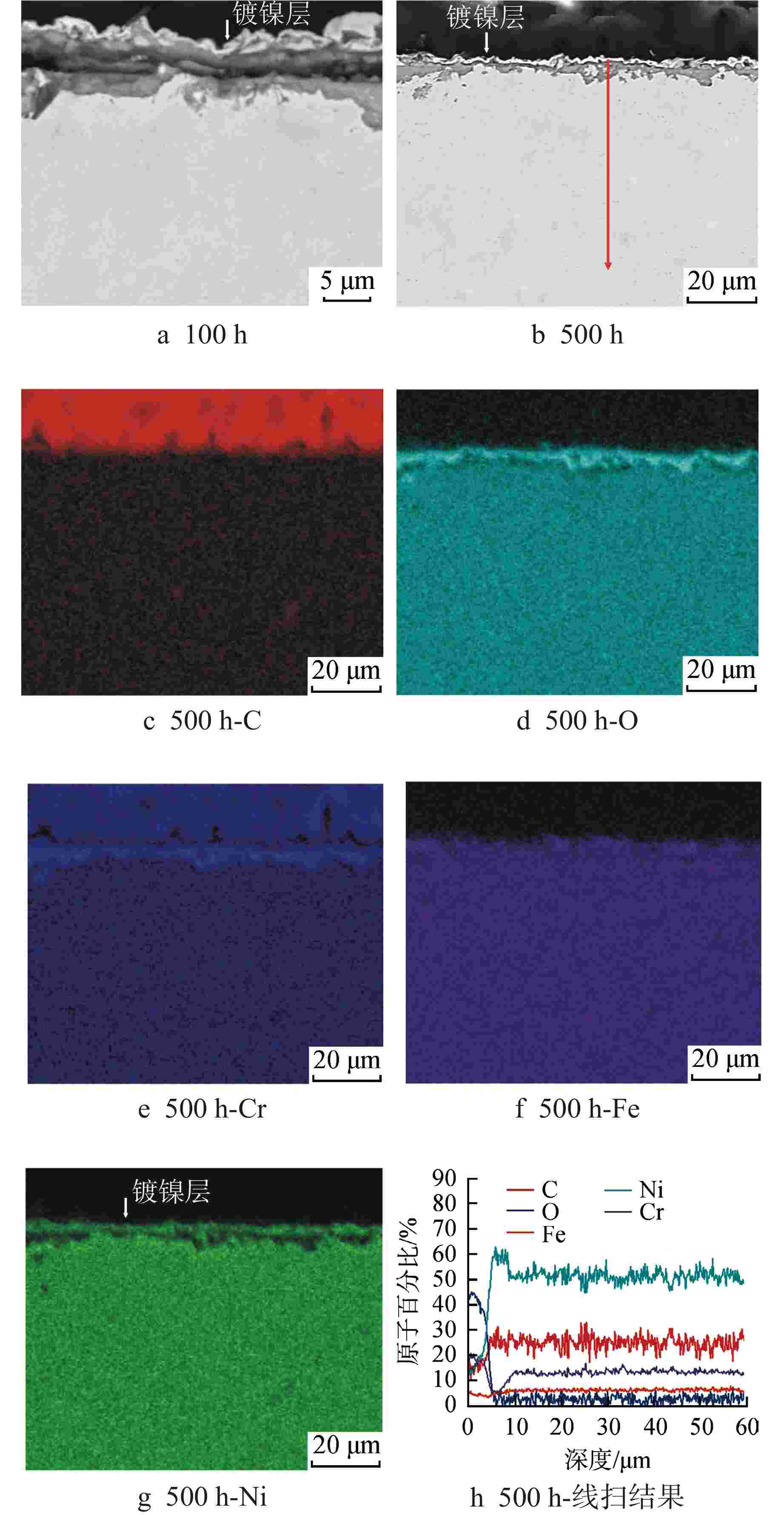
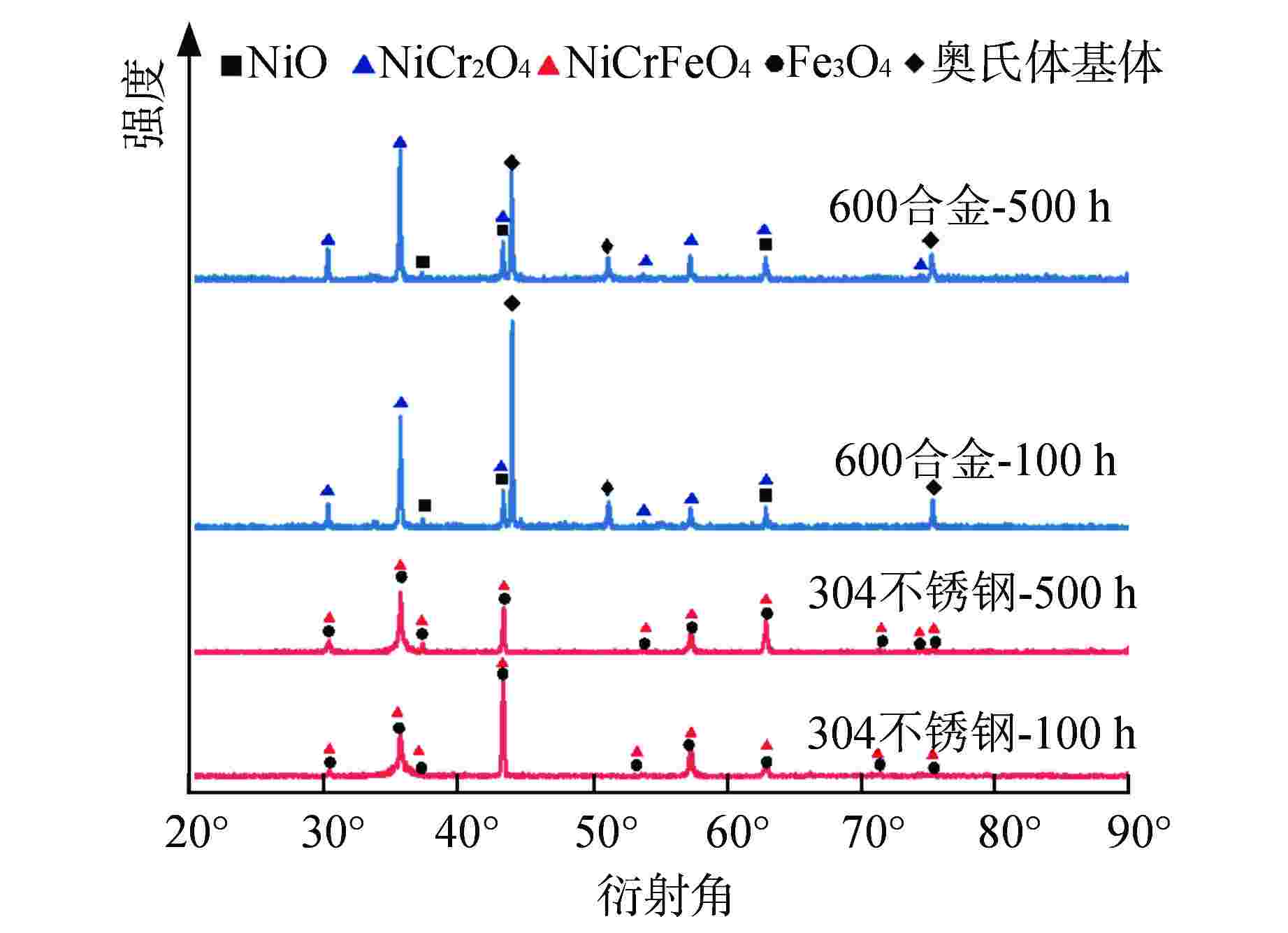
摘要: 为遴选可用于超临界二氧化碳核反应堆的结构材料,通过实验研究了应用于传统核反应堆中的两种合金(600合金和304不锈钢)在650℃、20 MPa的超临界二氧化碳环境中的均匀腐蚀行为,运用增重法评价了材料的腐蚀动力学规律,采用扫描电镜、能谱仪和X射线衍射仪分析了氧化膜形貌、结构和化学成分。结果表明,两种材料的腐蚀增重均服从抛物线生长规律,其中600合金的耐腐蚀性能优于304不锈钢;腐蚀500 h后,600合金表面氧化物厚度约为5 μm,主要成分为NiCr2O4,结构致密,具有保护性,其氧化膜及基体中均未发现明显渗碳行为;腐蚀500 h后,304不锈钢表面氧化膜可达约45 μm,为双层结构,外层为Fe3O4,内层为NiFeCrO4,结构疏松,发生显著渗碳现象。本研究揭示了上述材料在超临界二氧化碳中的腐蚀机理,为超临界二氧化碳核反应堆结构材料的选择提供了数据支持。Abstract: In order to select the structural materials that can be used in the supercritical carbon dioxide nuclear reactor, the uniform corrosion behavior of two kinds of alloys (600 alloy and 304 stainless steel) used in the traditional nuclear reactor in the supercritical carbon dioxide environment at 650℃ and 20 MPa is studied through experiments. The corrosion kinetics of the material is evaluated by weight gain method, and the morphology, structure and chemical composition of the oxide film are analyzed by scanning electron microscope, energy dispersive spectrometer and X-ray diffractometer. The results show that the corrosion weight gain of the two materials obeys the parabolic growth law, and the corrosion resistance of 600 alloy is better than that of 304 stainless steel. After 500 h corrosion, the oxide thickness on the surface of 600 alloy is about 5 μm, the main component is NiCr2O4, which is compact in structure and protective. No obvious carburization is found in its oxide film and matrix; After 500 h corrosion, the oxidation film on 304 stainless steel surface can reach about 45 μm. It is a double-layer structure, the outer layer is Fe3O4, and the inner layer is NiFeCrO4. The structure is loose, and significant carburization occurs. This study reveals the corrosion mechanism of the above materials in supercritical carbon dioxide, and provides valuable data support for the selection of structural materials for supercritical carbon dioxide nuclear reactors.
-
Key words:
- Supercritical carbon dioxide /
- 600 alloy /
- 304 stainless steel /
- Uniform corrosion /
- Carburization
-
表 1 实验材料化学成分
Table 1. Chemical Compositions of the Tested Materials
材料 元素质量分数/% C Si Mn Cr Fe Ni S P 304不锈钢 0.06 0.21 1.21 19.41 Bal. 9.35 0.007 0.018 600合金 0.06 0.22 0.23 16.36 8.50 Bal. 0.001 0.004 Bal.—Fe元素占比余量 表 2 不同标记区域处点扫结果
Table 2. Point Scanning Results at the Different Marked Areas
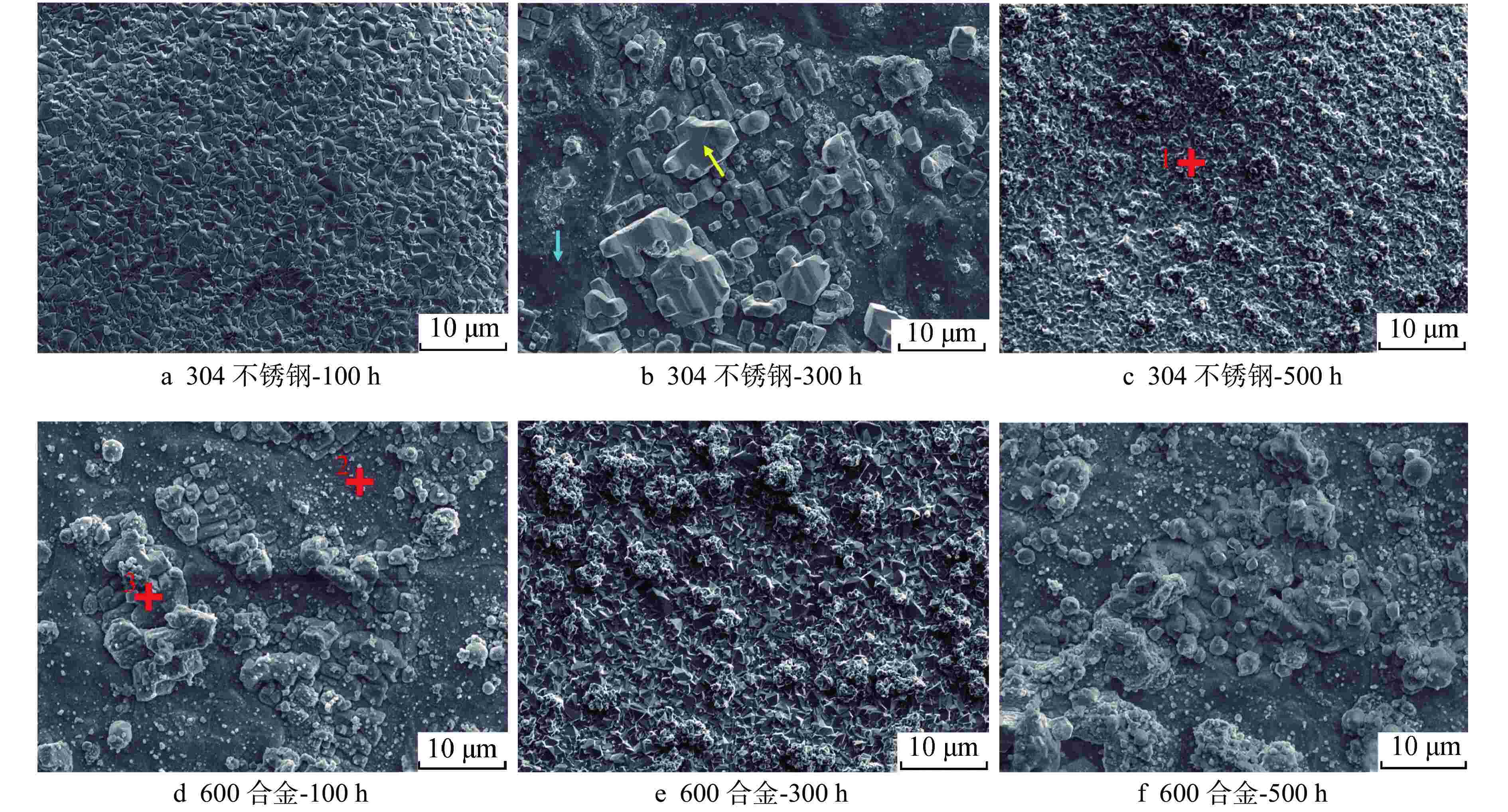
标记区域 原子百分比/% C O Fe Cr Ni 304不锈钢表面-1 36.2±2.0 42.8±0.3 20.6±2.1 0.3±0.2 0.1±0.03 600合金表面-2 40.9±1.1 38.3±2.4 1.8±0.2 7.9±0.1 11.0±1.2 600合金表面-3 62.3±1.8 15.1±0.1 0.7±0.1 0.6±0.2 21.4±1.9 -
[1] 黄彦平,王俊峰. 超临界二氧化碳在核反应堆系统中的应用[J]. 核动力工程,2012, 33(3): 21-27. doi: 10.3969/j.issn.0258-0926.2012.03.005 [2] 童家麟,赵寅丰. 超临界二氧化碳布雷顿循环研究进展[J]. 精细与专用化学品,2021, 29(1): 6-11. doi: 10.19482/j.cn11-3237.2021.01.02 [3] 吴攀,高春天,单建强. 超临界二氧化碳布雷顿循环在核能领域的应用[J]. 现代应用物理,2019, 10(3): 031202. [4] 赵煜,董自春,张羽,等. 超临界二氧化碳发电系统研究进展[J]. 热能动力工程,2019, 34(1): 11-16. doi: 10.16146/j.cnki.rndlgc.2019.01.002 [5] OH C, LILLO T, WINDES W, et al. Development of a supercritical Carbon Dioxide Brayton cycle: improving PBR efficiency and testing material compatibility: INEEL/EXT-04-02437[R]. Idaho Falls: Idaho National Lab, 2004. [6] 梁志远,桂雍,赵钦新. 超临界CO2动力循环高温材料腐蚀研究进展[J]. 动力工程学报,2021, 41(11): 910-917. [7] 刘蔚伟,杨鸿,姜峨,等. 超临界二氧化碳核能动力转换系统关键材料腐蚀行为研究[J]. 原子能科学技术,2021, 55(S2): 242-248. [8] 肖博,朱忠亮,李瑞涛,等. 超临界二氧化碳工质发电系统候选材料高温腐蚀研究现状与进展[J]. 热力发电,2020, 49(10): 30-37. doi: 10.19666/j.rlfd.202006155 [9] 刘珠,郭相龙,王鹏,等. 310S不锈钢在超临界二氧化碳中的腐蚀行为研究[J]. 核动力工程,2020, 41(S1): 183-187. doi: 10.13832/j.jnpe.2020.S1.0183 [10] CAO G, FIROUZDOR V, SRIDHARAN K, et al. Corrosion of austenitic alloys in high temperature supercritical carbon dioxide[J]. Corrosion Science, 2012, 60: 246-255. doi: 10.1016/j.corsci.2012.03.029 [11] HE L F, ROMAN P, LENG B, et al. Corrosion behavior of an alumina forming austenitic steel exposed to supercritical carbon dioxide[J]. Corrosion Science, 2014, 82: 67-76. doi: 10.1016/j.corsci.2013.12.023 [12] FIROUZDOR V, CAO G P, SRIDHARAN K, et al. Corrosion resistance of PM2000 ODS steel in high temperature supercritical carbon dioxide[J]. Materials and Corrosion, 2015, 66(2): 137-142. doi: 10.1002/maco.201307223 [13] Subramanian G O, Kim S H, Jang C. The carburization behavior of alloy 800HT in high temperature supercritical-CO2[J]. Materials Letters, 2021, 299: 130067. [14] ROUILLARD F, MARTINELLI L. Corrosion of 9Cr steel in CO2 at intermediate temperature III: modelling and simulation of void-induced duplex oxide growth[J]. Oxidation of Metals, 2012, 77(1): 71-83. [15] ZHU Z L, CHENG Y, XIAO B, et al. Corrosion behavior of ferritic and ferritic-martensitic steels in supercritical carbon dioxide[J]. Energy, 2019, 175: 1075-1084. doi: 10.1016/j.energy.2019.03.146 [16] BRITTAN A, MAHAFFEY J, ANDERSON M. The performance of Haynes 282 and its weld in supercritical CO2[J]. Materials Science and Engineering:A, 2019, 759: 770-777. doi: 10.1016/j.msea.2019.05.080 [17] MAHAFFEY J, ADAM D, BRITTAN A, et al. Corrosion of alloy Haynes 230 in high temperature supercritical carbon dioxide with oxygen impurity additions[J]. Oxidation of Metals, 2016, 86(5): 567-580. [18] PINT B A, BRESE R G, KEISER J R. Effect of pressure on supercritical CO2 compatibility of structural alloys at 750℃[J]. Materials and Corrosion, 2017, 68(2): 151-158. doi: 10.1002/maco.201508783 [19] FANG X R, YANG J H, SHAO Y R, et al. Quantitative prediction of stress corrosion crack propagation rate of small crack in alloy 600 for nuclear pressure vessels[J]. Rare Metal Materials and Engineering, 2019, 48(8): 2424-2431. [20] 闫红林. 近表面微观结构对国产核用304不锈钢高温水腐蚀行为的影响[D]. 合肥: 中国科学技术大学, 2021. [21] YOUNG D J. High temperature oxidation and corrosion of metals[M]. Amsterdam: Elsevier, 2008: 35. [22] GUI Y, LIANG Z Y, ZHAO Q X. Corrosion and carburization behavior of heat-resistant steels in a high-temperature supercritical carbon dioxide environment[J]. Oxidation of Metals, 2019, 92(1): 123-136. [23] CHEN H S, KIM S H, KIM C, et al. Corrosion behaviors of four stainless steels with similar chromium content in supercritical carbon dioxide environment at 650℃[J]. Corrosion Science, 2019, 156: 16-31. doi: 10.1016/j.corsci.2019.04.043 [24] GUO X L, CHEN K, GAO W H, et al. Corrosion behavior of alumina-forming and oxide dispersion strengthened austenitic 316 stainless steel in supercritical water[J]. Corrosion Science, 2018, 138: 297-306. doi: 10.1016/j.corsci.2018.04.026 [25] ROUILLARD F, MOINE G, MARTINELLI L, et al. Corrosion of 9Cr steel in CO2 at intermediate temperature I: mechanism of void-induced duplex oxide formation[J]. Oxidation of Metals, 2012, 77(1-2): 27-55. doi: 10.1007/s11085-011-9271-5 [26] YOUNG D J, ZHANG J Q. Alloy corrosion by Hot CO2 gases[J]. JOM, 2018, 70(8): 1493-1501. doi: 10.1007/s11837-018-2944-7 [27] LIANG Z Y, GUI Y, WANG Y G, et al. Corrosion performance of heat-resisting steels and alloys in supercritical carbon dioxide at 650℃ and 15 MPa[J]. Energy, 2019, 175: 345-352. doi: 10.1016/j.energy.2019.03.014 [28] LEE H J, KIM H, KIM S H, et al. Corrosion and carburization behavior of chromia-forming heat resistant alloys in a high-temperature supercritical-carbon dioxide environment[J]. Corrosion Science, 2015, 99: 227-239. doi: 10.1016/j.corsci.2015.07.007 [29] MAHAFFEY J T. Effect of partial pressure of oxygen and activity of carbon on the corrosion of high temperature alloys in s-CO2 environments[D]. Madison: The Univ. of Wisconsin, 2017: 63-64. [30] SRIDHARAN K. Corrosion of structural materials for advanced supercritical carbon-dioxide Brayton cycle: 13-4900[R]. Madison: University of Wisconsin, 2017. [31] OLEKSAK R P, HOLCOMB G R, CARNEY C S, et al. Effect of surface finish on high-temperature oxidation of steels in CO2, supercritical CO2, and air[J]. Oxidation of Metals, 2019, 92(5-6): 525-540. doi: 10.1007/s11085-019-09938-6 [32] ROTHMAN S J, NOWICKI L J, MURCH G E. Self-diffusion in austenitic Fe-Cr-Ni alloys[J]. Journal of Physics F:Metal Physics, 1980, 10(3): 383-398. doi: 10.1088/0305-4608/10/3/009 [33] SABIONI A C S, HUNTZ A M, SILVA F, et al. Diffusion of iron in Cr2O3: polycrystals and thin films[J]. Materials Science and Engineering:A, 2005, 392(1-2): 254-261. doi: 10.1016/j.msea.2004.09.033 [34] COX M G C, MCENANEY B, SCOTT V D. A chemical diffusion model for partitioning of transition elements in oxide scales on alloys[J]. The Philosophical Magazine:A Journal of Theoretical Experimental and Applied Physics, 1972, 26(4): 839-851. doi: 10.1080/14786437208226960 [35] ROUILLARD F, FURUKAWA T. Corrosion of 9-12Cr ferritic-martensitic steels in high-temperature CO2[J]. Corrosion Science, 2016, 105: 120-132. doi: 10.1016/j.corsci.2016.01.009 [36] ROUILLARD F, MOINE G, TABARANT M, et al. Corrosion of 9Cr steel in CO2 at intermediate temperature II: mechanism of carburization[J]. Oxidation of Metals, 2012, 77(1-2): 57-70. doi: 10.1007/s11085-011-9272-4 [37] DE RECA E W, PAMPILLO C. Self-diffusion of Ni in Ni-Fe alloys[J]. Acta Metallurgica, 1967, 15(8): 1263-1268. doi: 10.1016/0001-6160(67)90001-6 [38] HÄNSEL M, QUADAKKERS W J, YOUNG D J. Role of water vapor in chromia-scale growth at low oxygen partial pressure[J]. Oxidation of Metals, 2003, 59(3-4): 285-301. [39] ASTEMAN H, SVENSSON J E, JOHANSSON L G, et al. Indication of chromium oxide hydroxide evaporation during oxidation of 304L at 873 K in the presence of 10% water vapor[J]. Oxidation of Metals, 1999, 52(1-2): 95-111. [40] Colwell J A, Rapp R A. Reactions of Fe-Cr and Ni-Cr alloys in CO/CO2 gases at 850 and 950℃[J]. Metallurgical Transactions A, 1986, 17(6): 1065-1074. -






 下载:
下载: