Effect of Fe+Cr and Si Contents on Corrosion Resistance of Zircaloy-4
-
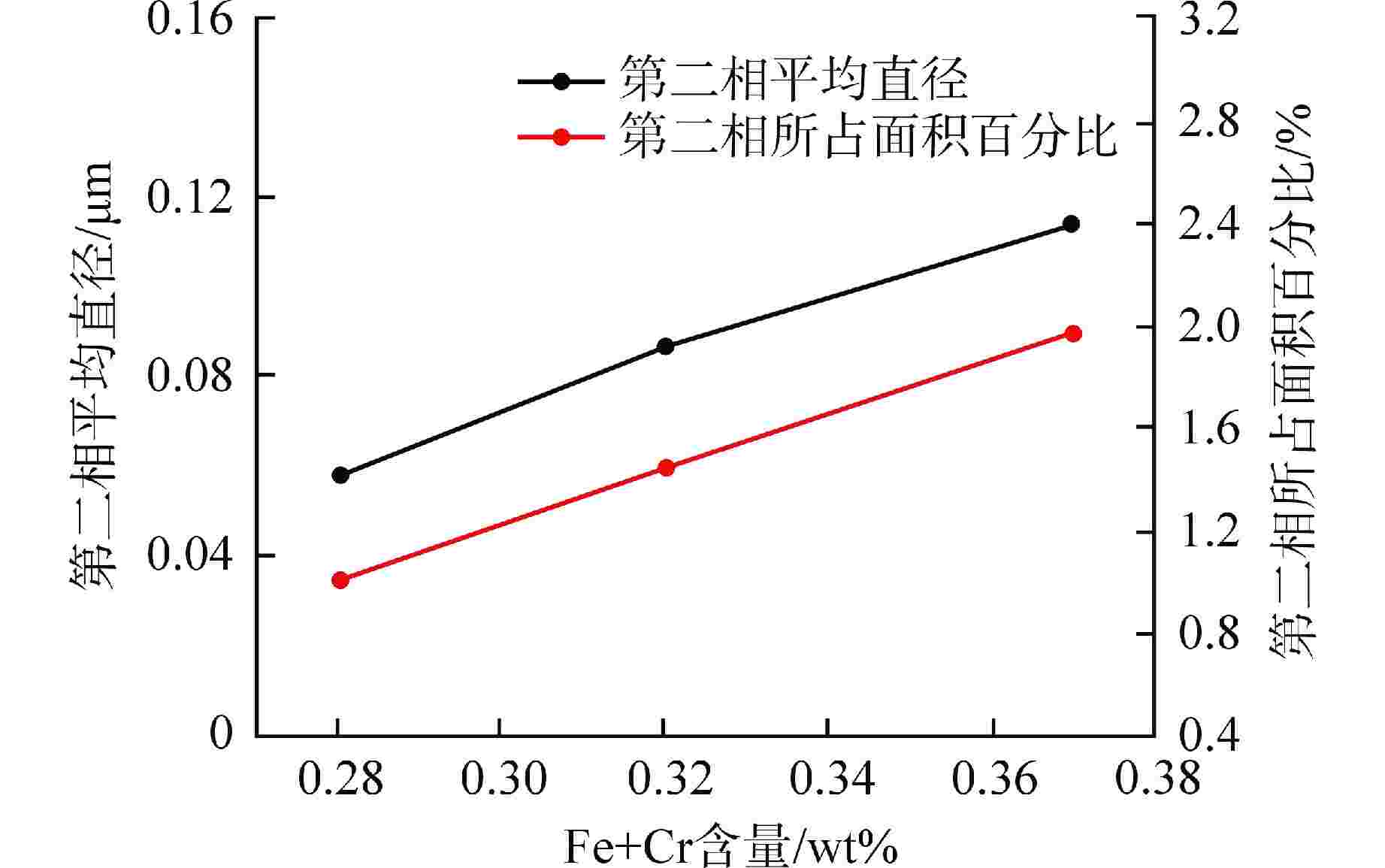
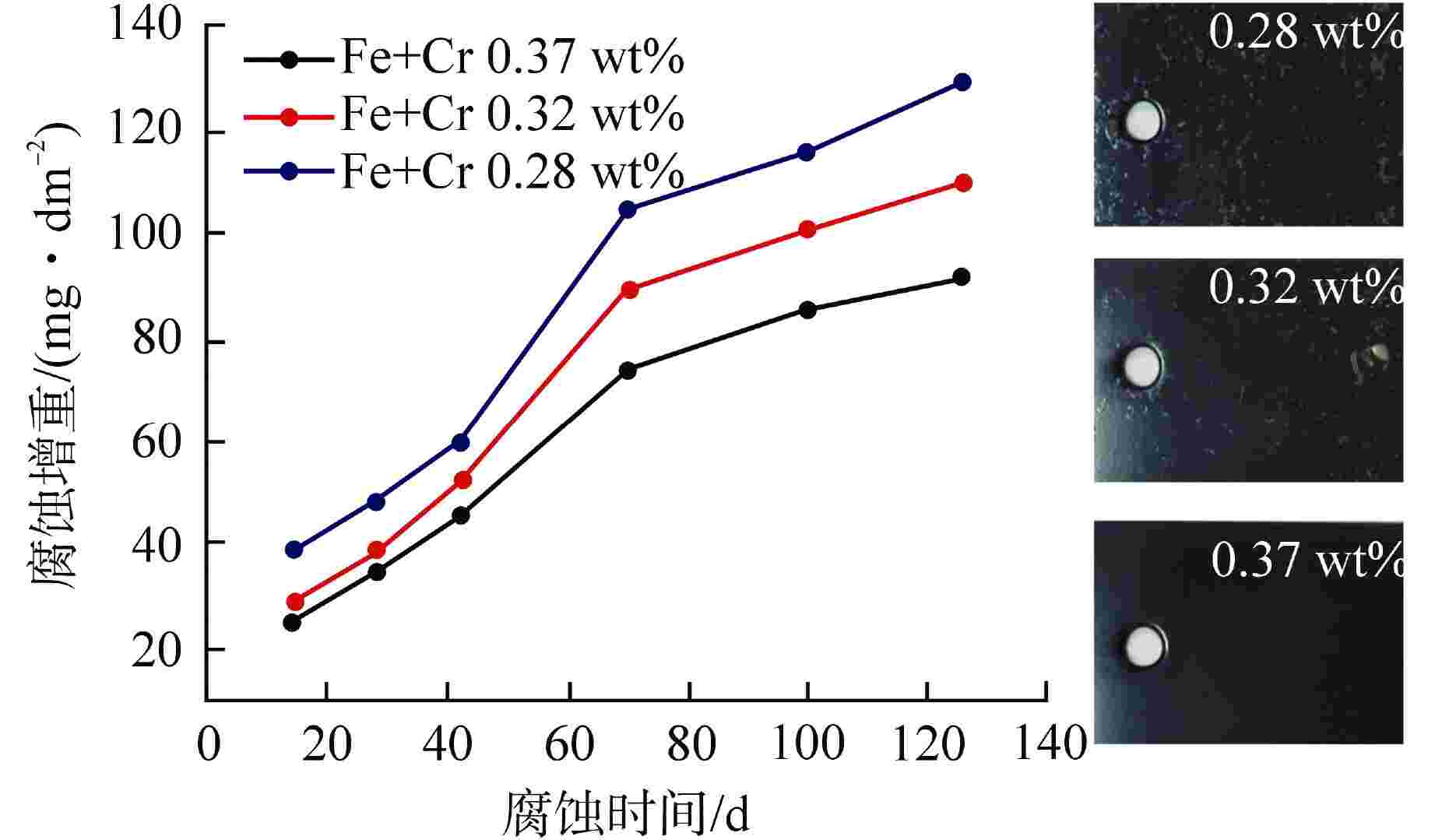
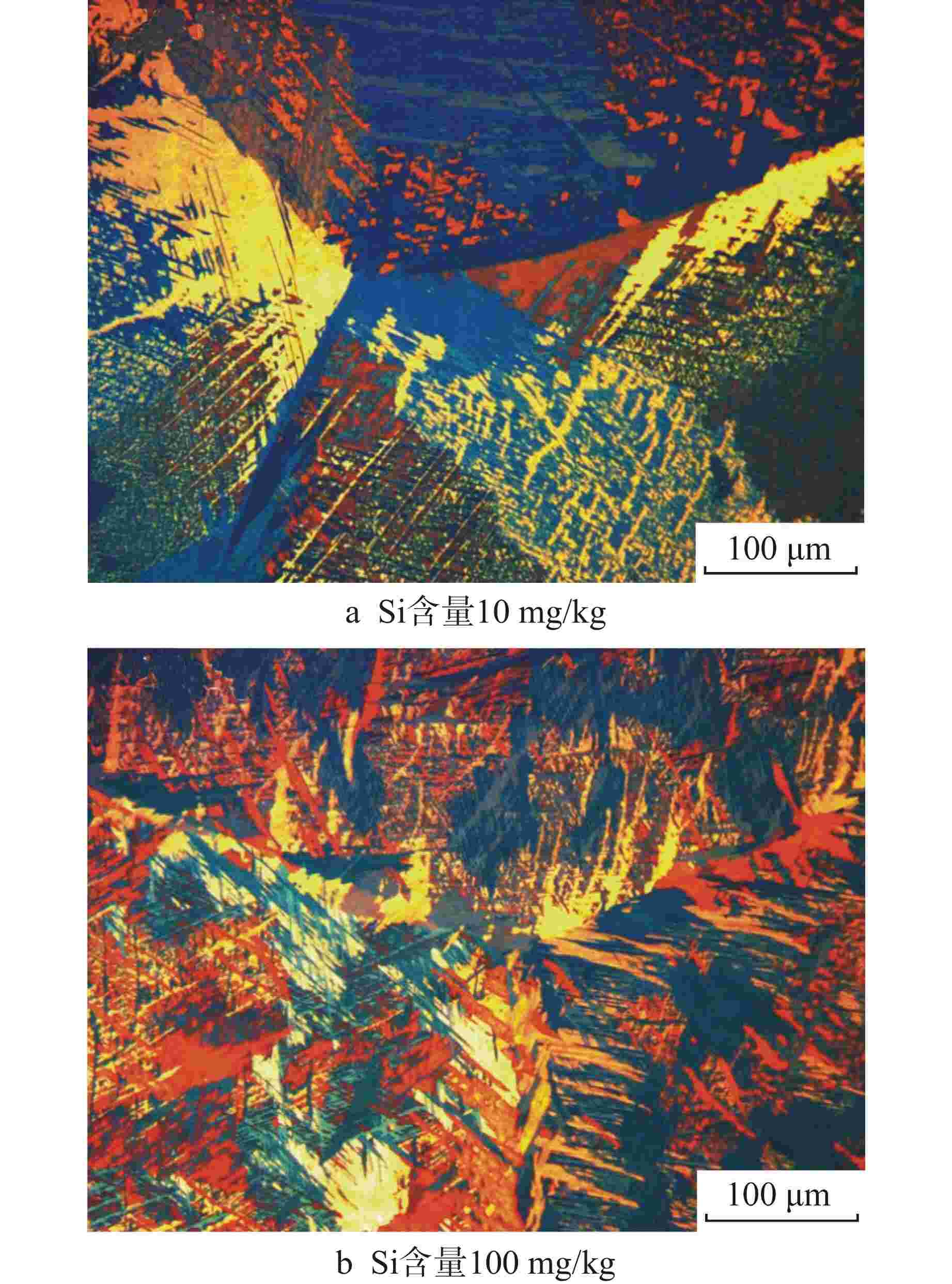
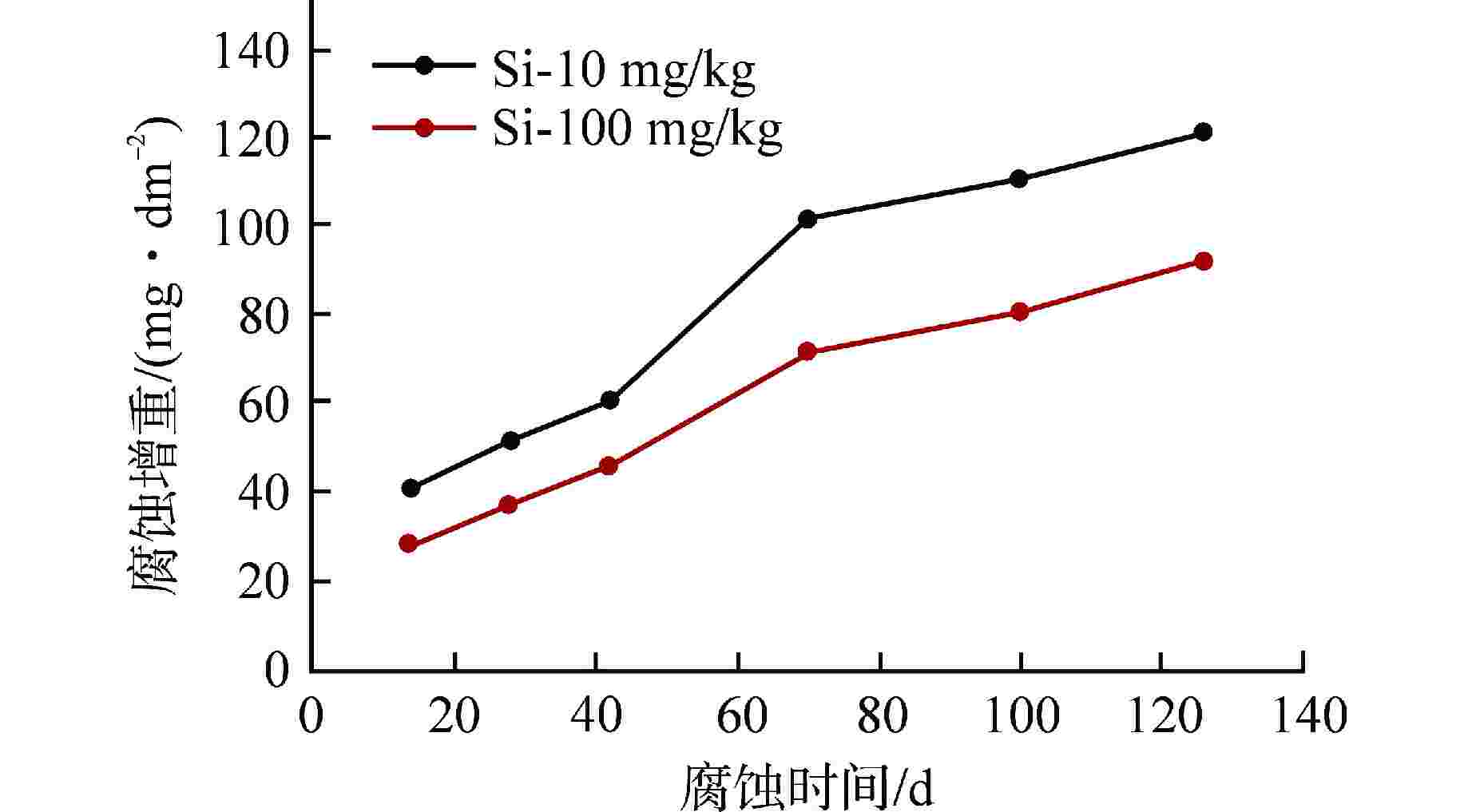
摘要: 为了优化国产Zr-4合金的耐腐蚀性能,在420℃、10.3 MPa的高温高压水蒸气加速腐蚀条件下,研究了合金元素Fe+Cr以及杂质元素Si对国产Zr-4合金耐腐蚀性能的影响。结果表明:在美国材料实验学会(ASTM)规定的Fe+Cr含量范围内(0.28 wt%~0.37 wt%,wt%表示质量分数),Fe+Cr含量越高,析出的第二相数目越多、尺寸越大,越有利于材料耐腐蚀性能提升。在420℃水蒸气中腐蚀126 d后,当Fe+Cr含量从0.28 wt%增加到0.37 wt%时,Zr-4合金的腐蚀增重降低约30%。Zr-4合金在1100℃淬火时,当Si含量低至10 mg/kg时,形成粗大的平行板条结构;而增加Si含量到100 mg/kg后,析出的细小Zr3Si为α相提供形核位置,使得组织中出现网篮结构和细小的平行板条结构。网篮结构能促使组织中第二相分布更均匀弥散,因此,高Si含量的Zr-4合金表现出更优良的耐腐蚀性能。Abstract: In order to optimize the corrosion resistance of domestic Zr-4 alloy, the effects of alloying element Fe+Cr and impurity element Si on the corrosion resistance of domestic Zr-4 alloy were studied under the accelerated corrosion condition of high temperature and high pressure steam at 420℃ and 10.3 MPa. The results show that within the range of Fe+Cr content specified by ASTM (0.28 wt%-0.37 wt%, with wt% representing mass percentage), the higher the Fe+Cr content, the larger the number and size of the precipitated second phases, which is beneficial to the improvement of the corrosion resistance of the material. When the content of Fe+Cr increases from 0.28 wt% to 0.37 wt%, the corrosion weight gain of Zr-4 alloy decreases by about 30% after 126 d of corrosion in steam at 420℃. When the Si content of Zr-4 alloy is as low as 10 mg/kg during quenching at 1100℃, the coarse parallel-plate structure is formed. When the Si content is increased to 100 mg/kg, the precipitated fine Zr3Si provides nucleation sites for α phase, which leads to the appearance of basketweave structure and fine parallel-plate structure in the microstructure. The basketweave structure can promote the distribution of the second phase in the structure to be more uniform and diffuse, so the Zr-4 alloy with high Si content shows better corrosion resistance.
-
Key words:
- Zr-4 alloy /
- Fe+Cr content /
- Si content /
- Corrosion resistance
-
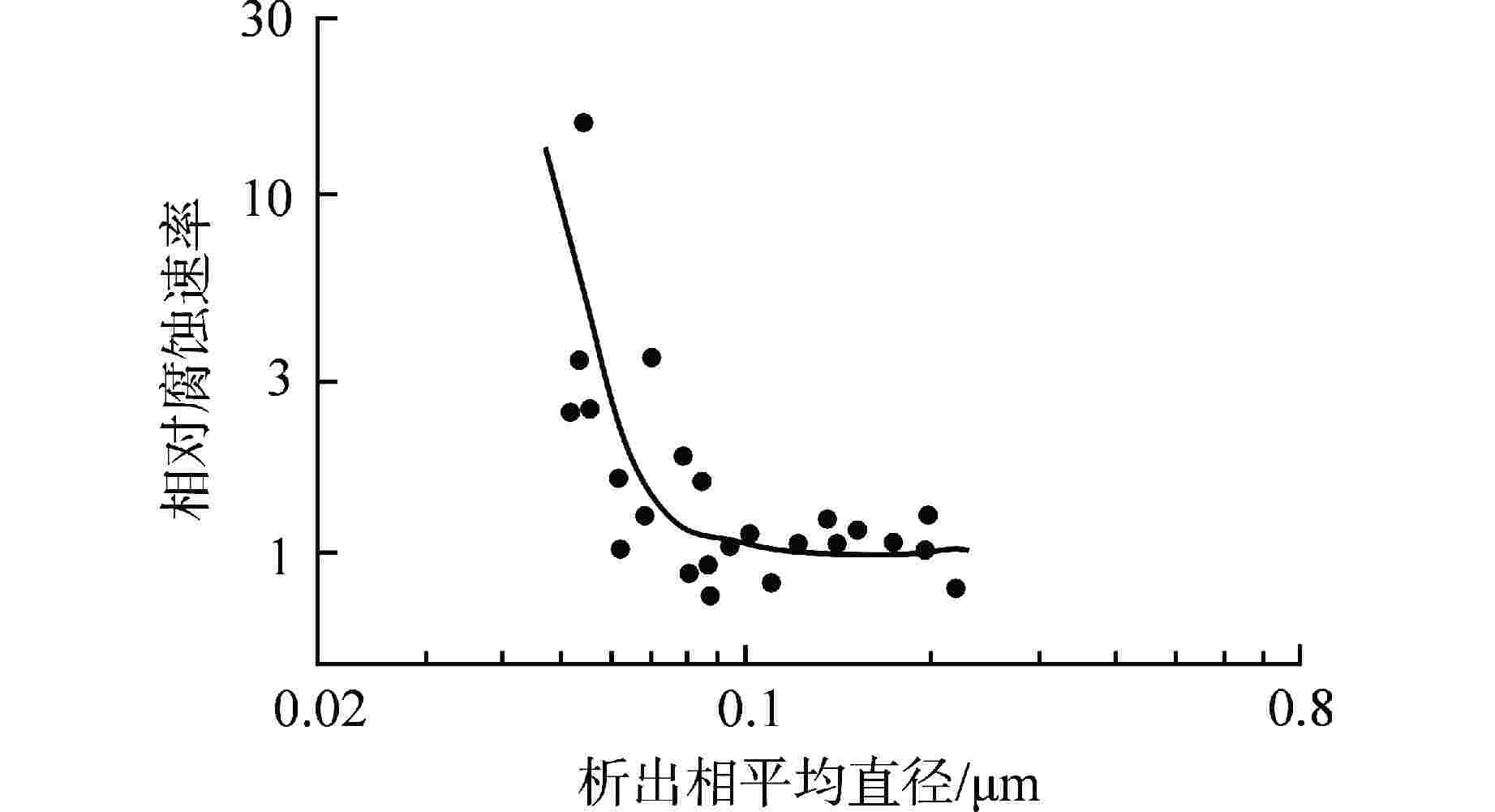
图 5 锆合金第二相粒子平均直径与腐蚀速率的依赖关系[21]
Figure 5. Dependence of Mean Diameter of Zircaloy Second Phase Particles on Corrosion Rate
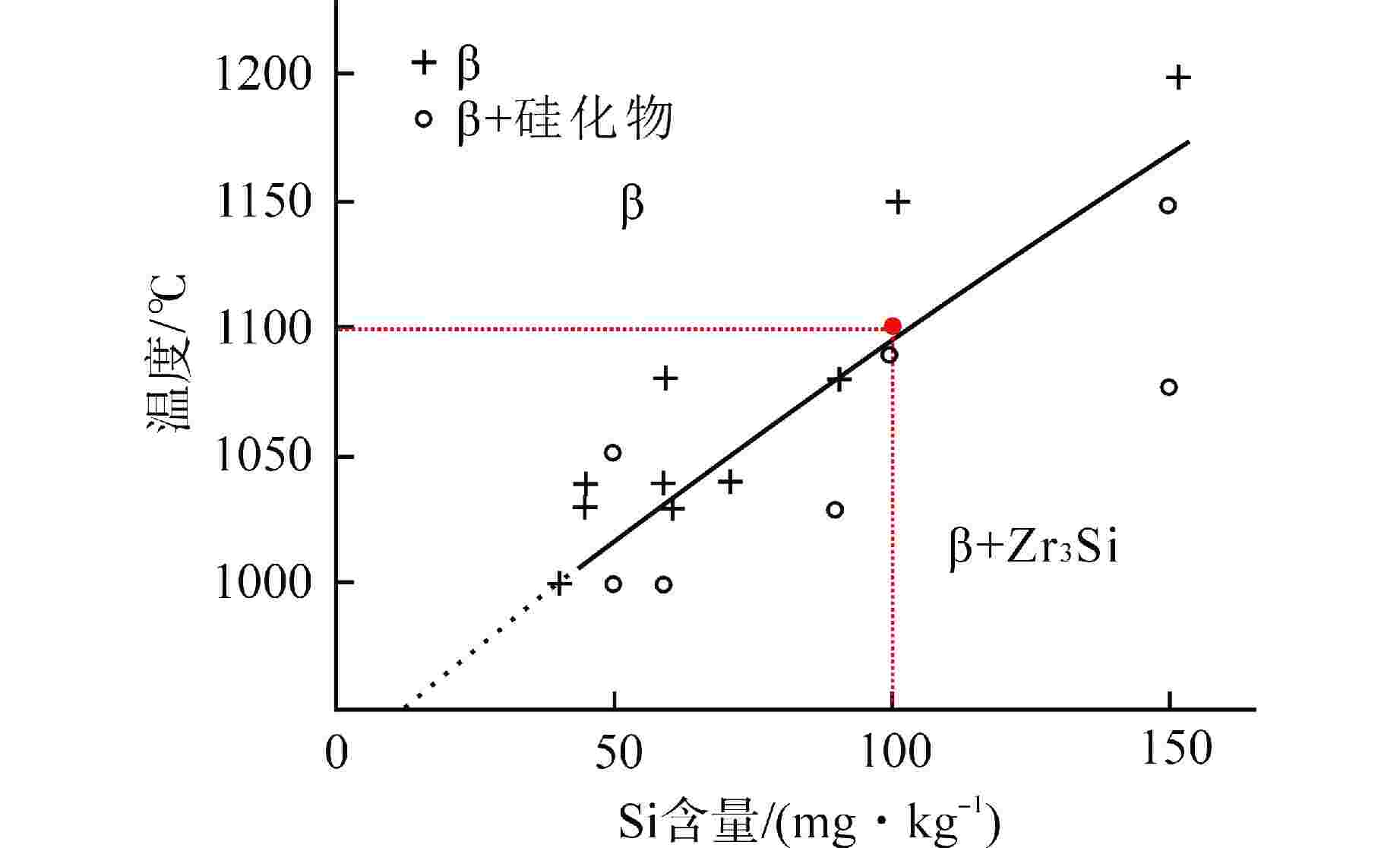
图 7 锆合金中β/β+硅化物物相转变图[22]
Figure 7. Phase Transition Diagram of β/β+silicide in Zircaloy
表 1 ASTM B811规定的Zr-4合金主要成分范围[16]
Table 1. Primary Element Range of Zr-4 Alloy Specified by ASTM B811[16]
合金成分 Sn Fe+Cr C Si 标准范围/wt% 1.2~1.7 0.28~0.37 <0.027 <0.012 wt%表示质量分数 表 2 样品成分
Table 2. Composition of the Samples
样品编号 Fe+Cr
含量/wt%Fe/Cr
比值C含量/
(mg·kg−1)P含量/
(mg·kg−1)Si含量/
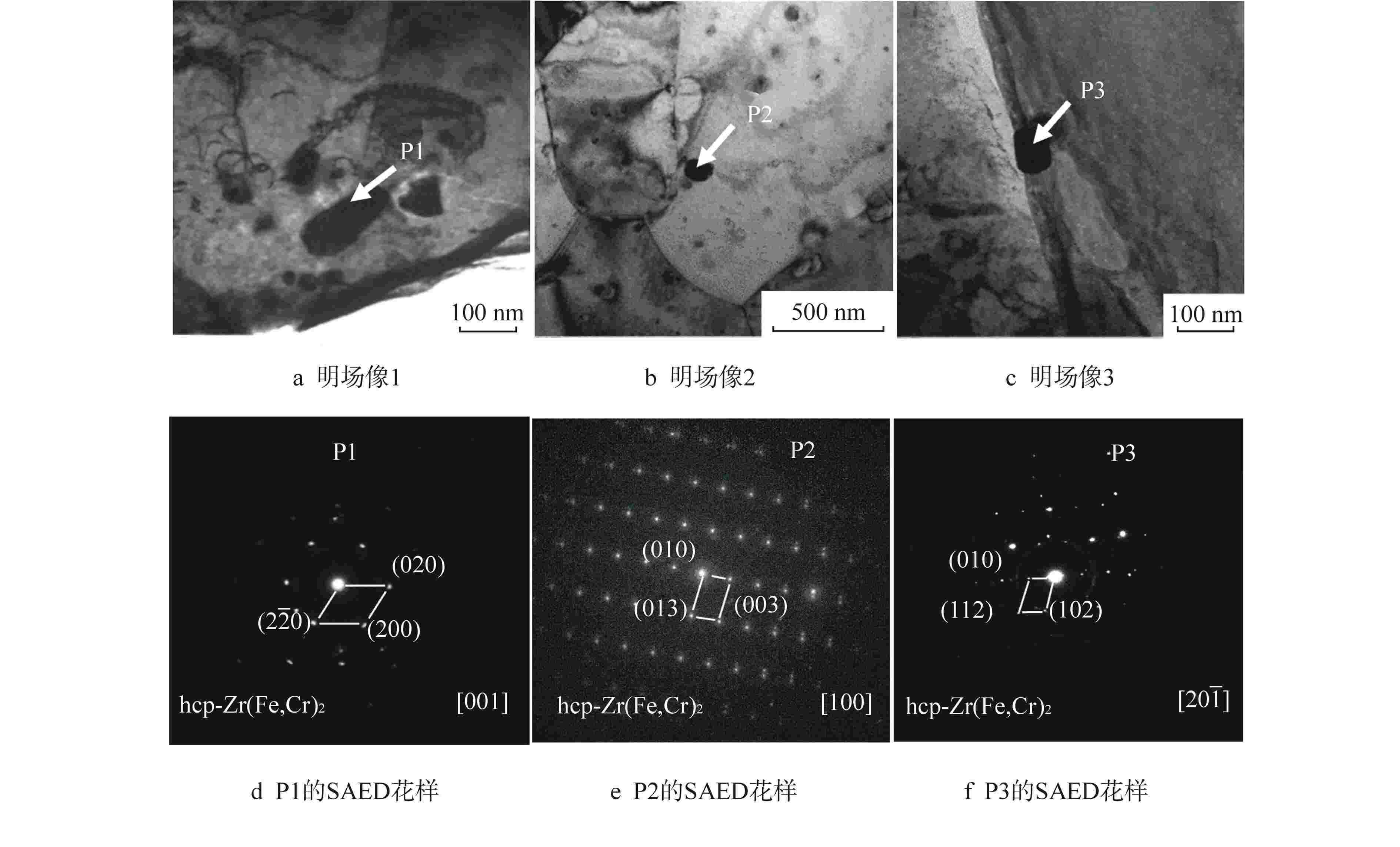
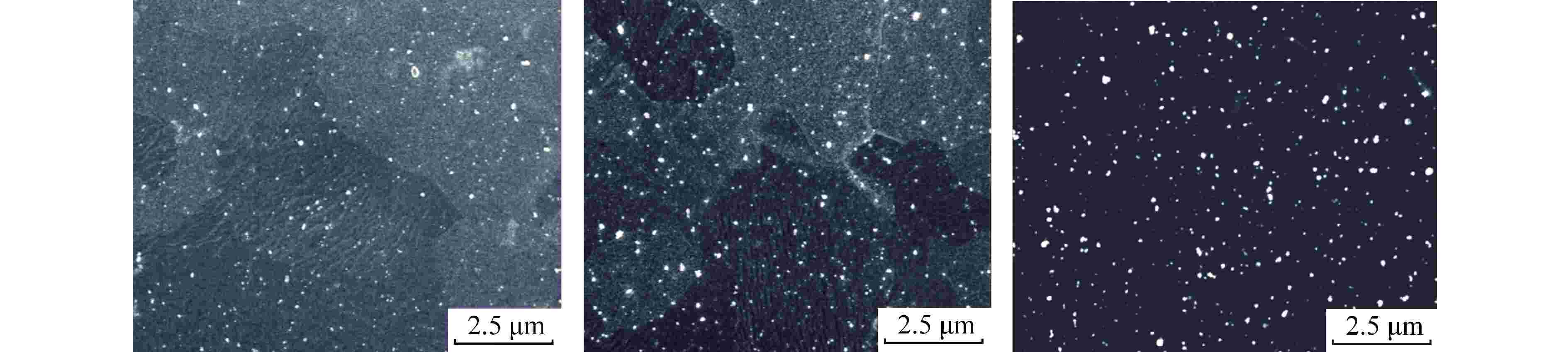
(mg·kg−1)样品1 0.28 2.1 150 <10 100 样品2 0.32 1.9 150 <10 100 样品3 0.37 2.1 150 <10 100 样品4 0.32 1.9 30 <10 10 样品5 0.32 1.9 30 <10 100 表 3 Zr-4合金样品中第二相P1、 P2、 P3的EDS分析(对应图3)
Table 3. EDS Analyses of Second Phase Particles P1, P2, P3 in the Zr-4 Sample (Corresponding to Fig. 3)
第二相 化学成分/at% Zr Fe Cr Sn Fe/Cr比值 P1 47.29 34.22 18.24 0.25 1.88 P2 45.55 34.76 19.47 0.22 1.79 P3 51.34 31.17 17.38 0.11 1.79 at%表示原子百分比 -
[1] LIU W Q, LI Q, ZHOU B X, et al. Effect of heat treatment on the microstructure and corrosion resistance of a Zr-Sn-Nb-Fe-Cr alloy[J]. Journal of Nuclear Materials, 2005, 341(2-3): 97-102. doi: 10.1016/j.jnucmat.2005.01.007 [2] 姚美意,邹玲红,谢兴飞,等. 添加Bi对Zr-4合金在400℃/10.3MPa过热蒸汽中耐腐蚀性能的影响[J]. 金属学报,2012, 48(9): 1097-1102. [3] 李强,周邦新,姚美意,等. 锆合金在550℃,25MPa超临界水中的腐蚀行为[J]. 稀有金属材料与工程,2007, 36(8): 1358-1361. [4] 朱伟,王桢,吴璐,等. 新锆合金不同热处理条件下第二相结构及成分变化研究[J]. 现代应用物理,2023, 14(1): 010801. [5] 姚美意,周邦新,李强,等. 第二相对Zr-4合金在400℃过热蒸汽中腐蚀吸氢行为的影响[J]. 稀有金属材料与工程,2007, 36(11): 1915-1919. doi: 10.3321/j.issn:1002-185x.2007.11.008 [6] 王德鹏,李毅丰,梁雪,等. 压水堆燃料包壳锆合金中第二相的腐蚀行为研究进展[J]. 稀有金属材料与工程,2023, 52(2): 753-762. [7] 周邦新. 改善锆合金耐腐蚀性能的概述[J]. 金属热处理学报,1997, 18(3): 8-15. [8] 陈鑫,李中奎,周军,等. 合金元素对锆合金耐腐蚀性能的影响概述[J]. 热加工工艺,2015, 44(2): 14-16. [9] CHARQUET D. Influence of precipitate density on the nodular corrosion resistance of Zr-Sn-Fe-Cr alloys at 500℃[J]. Journal of Nuclear Materials, 2001, 288(2-3): 237-240. doi: 10.1016/S0022-3115(00)00728-5 [10] 栾佰峰,薛姣姣,柴林江,等. 冷却速率及杂质元素对锆合金β→α转变组织的影响[J]. 稀有金属材料与工程,2013, 42(12): 2636-2640. [11] 刘琼,谢梦,李宇力,等. 浅析锆合金β淬火组织差异[J]. 有色金属加工,2021, 50(1): 32-35. [12] ÖKVIST G, KÄLLSTRÖM K. The effect of zirconium carbide on the β→α transformation structur in zircaloy[J]. Journal of Nuclear Materials, 1970, 35(3): 316-321. doi: 10.1016/0022-3115(70)90215-1 [13] QUACH V, NORTHWOOD D O. Influence of the phosphorus impurity content on the microstructure of Zircaloy-4 air cooled from the high temperature beta phase region[J]. Metallography, 1984, 17(2): 191-201. doi: 10.1016/0026-0800(84)90022-3 [14] QUACH V. The influence of the impurity content and cooling rate on the microstructure of Zircaloy-4 nuclear fuel cladding[D]. Windsor: University of Windsor, 1984. [15] FONG W L, NORTHWOOD D O. Microstructure-impurity content relationships in Zircaloy-4 nuclear fuel sheathing[C]//14th Annual Technical Meeting of the International Metallographic Society. San Francisco: Microstructural Science, 1982: 123-130. [16] ASTM International. Standard specification for wrought zirconium alloy seamless tubes for nuclear reactor fuel cladding: ASTM B811-13[S]. United States: ASTM International, 2022. [17] 柴林江,栾佰峰,周宇,等. 锆合金第二相研究述评(Ⅰ): Zircaloys合金[J]. 中国有色金属学报,2012, 22(6): 1594-1604. [18] 周邦新,李聪,黄德诚. Zr(Fe,Cr)2金属间化合物的氧化[J]. 核动力工程,1993, 14(2): 149-153, 190. [19] PÊCHEUR D. Oxidation of β-Nb and Zr(Fe, V)2 precipitates in oxide films formed on advanced Zr-based alloys[J]. Journal of Nuclear Materials, 2000, 278(2-3): 195-201. doi: 10.1016/S0022-3115(99)00253-6 [20] 杨忠波,赵文金. 锆合金耐腐蚀性能及氧化特性概述[J]. 材料导报,2010, 24(9): 120-125. [21] 刘建章. 核结构材料[M]. 北京: 化学工业出版社,2007:26. [22] CHARQUET D, ALHERITIERE E. Influence of impurities and temperature on the microstructure of zircaloy-2 and zircaloy-4 after the Beta → Alpha phase transformation[M]//ADAMSON R B, VAN SWAM L F P. Zirconium in the Nuclear Industry. Philadelphia: ASTM, 1987: 284-291. [23] COX B, KRITSKY V, LEMAIGNAN C, et al. Waterside corrosion of zirconium alloys in nuclear power plants: IAEA-TECDOC-996[R]. Vienna: IAEA, 1998: 124. [24] SELL HJ, Trapp-Pritsching S, Garzarolli F. Effect of alloying elements and impurities on in-BWR corrosion of Zironium alloys[J]. Journal of ASTM International, 2006, 3(1):404-417. -





 下载:
下载: